Key Points
Sirolimus monotherapy is a safe and effective steroid-sparing agent, improving autoimmune cytopenias in highly refractory patients.
Sirolimus is particularly active in ALPS and should be an early therapy option for patients who require chronic therapy.
Abstract
Patients with autoimmune multilineage cytopenias are often refractory to standard therapies requiring chronic immunosuppression with medications with limited efficacy and high toxicity. We present data on 30 patients treated on a multicenter prospective clinical trial using sirolimus as monotherapy. All children (N = 12) with autoimmune lymphoproliferative syndrome (ALPS) achieved a durable complete response (CR), including rapid improvement in autoimmune disease, lymphadenopathy, and splenomegaly within 1 to 3 months of starting sirolimus. Double-negative T cells were no longer detectable in most, yet other lymphocyte populations were spared, suggesting a targeted effect of sirolimus. We also treated 12 patients with multilineage cytopenias secondary to common variable immunodeficiency (CVID), Evans syndrome (ES), or systemic lupus erythematosus (SLE), and most achieved a CR (N = 8), although the time to CR was often slower than was seen in ALPS. Six children with single-lineage autoimmune cytopenias were treated and only 2 responded. Sirolimus was well tolerated with very few side effects. All of the responding patients have remained on therapy for over 1 year (median, 2 years; range, 1 to 4.5 years). In summary, sirolimus led to CR and durable responses in a majority of children with refractory multilineage autoimmune cytopenias. The responses seen in ALPS patients were profound, suggesting that sirolimus should be considered as a first-line, steroid-sparing treatment of patients needing chronic therapy. The results in other multilineage autoimmune cytopenia cohorts were encouraging, and sirolimus should be considered in children with SLE, ES, and CVID. This trial was registered at www.clinicaltrials.gov as #NCT00392951.
Introduction
Autoimmune cytopenias are a heterogeneous yet related group of disorders defined by immune-mediated destruction of hematopoietic cells. Patients can have single or multilineage disease and can be primary (formerly classified as idiopathic) or secondary to other illnesses.1,2 Unlike children with single-lineage autoimmune cytopenias, those with multilineage autoimmune cytopenias often have chronic, therapy-refractory disease.1,3-8 Unfortunately, few effective and well-tolerated chronic therapies exist.4,8
Autoimmune lymphoproliferative syndrome (ALPS) is a disorder of abnormal lymphocyte survival caused by a failure of the FAS apoptotic pathway to maintain lymphocyte homeostasis.3,4,7 Due to the persistence of autoreactive cells, ALPS patients develop chronic nonmalignant lymphadenopathy, splenomegaly, multilineage cytopenias, and an increased risk of lymphoma.1 Over 70% of patients with ALPS have identifiable mutations in FAS pathway genes.4 Most patients have germline (60% to 70%; ALPS-FAS) or somatic mutations in FAS (10%). Rarely, ALPS patients have mutations in FASL (<1%) and CASP10 (2% to 3%).9,10 Approximately one-third of patients with ALPS have yet undetermined genetic defects (ALPS-U).11
Treatment of patients with ALPS varies significantly, with no consensus on the management. We have previously demonstrated that treatment with the mammalian target of rapamycin inhibitor, sirolimus (Rapamune) led to a complete response (CR) in a small retrospective cohort of 5 children with corticosteroid refractory ALPS.5 Based on that study, we opened a prospective multi-institutional clinical trial (#NCT00392951) using sirolimus for children with treatment-refractory ALPS. After we found an early efficacy signal, we broadened the inclusion criteria to any child with autoimmune cytopenias who failed or was intolerant to standard immunosuppressive therapy (corticosteroids and/or IV immunoglobulin G [IVIgG]). We report the results of 30 patients treated on the trial and found that sirolimus is well tolerated for both short- and long-term use. The majority of the patients had a CR and durable response with few to no adverse effects. We also monitored immune function on a subset of the patients and found sirolimus was effective and did not suppress B- or T-cell numbers or function in the majority of patients with secondary autoimmune cytopenias. We would propose that sirolimus should be considered as a first- or second-line agent in certain patients with chronic and refractory autoimmune cytopenias.
Materials and methods
Patients
Patients aged ≥12 months to <40 years of age, with a diagnosis of autoimmune cytopenias requiring medications, were eligible. “Autoimmune” was defined by meeting one of the following criteria: documented autoantibody (positive direct antiglobulin test, antineutrophil, or antiplatelet antibody), or a history of documented clinical response to immunosuppression. Patients must have at least one of the following conditions: autoimmune neutropenia, autoimmune thrombocytopenia, or autoimmune hemolytic anemia (AIHA). The diagnosis of ALPS was made based on revised criteria.11
Study design
This was a multicenter, prospective, open label clinical trial to evaluate the safety and efficacy of sirolimus in children and young adults with autoimmune cytopenias who were either refractory to standard therapy or had significant toxicity from standard treatments. A total of 15 different treating institutions from across the United States were included in this study. Because autoimmune cytopenias are rare, subjects traveled to the Children’s Hospital of Philadelphia or the Children’s Hospital of Wisconsin for consent, but all study procedures including prescription of sirolimus, laboratory monitoring, and physical examinations occurred at the referring primary institutions. All patients on the study gave written informed consent. The study was approved by local institutional review boards, and was conducted in accordance with the provision of the Declaration of Helsinki and Good Clinical Practice guidelines.
Treatment
Sirolimus was given at a dose of 2 to 2.5 mg/m2 per day rounded to the nearest 0.5 (liquid) or 1 mg (tablet), starting on day 1 (maximum initial dose of 4 mg/day). Detailed description regarding the recommended dose adjustment is included in supplemental Methods on the Blood Web site. The anticipated length of treatment with sirolimus was 6 months, however, patients with a favorable response were given the option to continue treatment with continued serial follow-up to monitor for efficacy and toxicity.
Evaluation of disease responses and toxicity monitoring
The response criteria for autoimmune cytopenia, lymphadenopathy, and splenomegaly are included in supplemental Table 1. Evaluation of response was monitored by blood counts measured every 2 weeks until trough sirolimus goal levels were reached, then spaced to every 3 months. As additional measures of efficacy, immune cell assays and measures of immune function were performed in a portion of patients, including T-cell subsets (CD4+, CD8+, and double-negative T cells [DNTs]), quantitative IgG, and mitogen assays, including phytohemagglutinin A, concanavalin A, and poke weed mitogen. All patients underwent periodic biochemical analysis in order to evaluate organ toxicity. All adverse events were assessed at each patient visit and were graded according to the National Cancer Institute Common Toxicity Criteria for Adverse Events (version 4.0). Assessments of efficacy and safety were conducted formally every 3 months, and followed long-term.
Statistical analysis
Descriptive statistics were reported in terms of absolute frequencies and percentage for qualitative data. Distribution of data regarding continuous variables was described in terms of a median value. Comparison of laboratory values of pre- and post-sirolimus was performed using the Wilcoxon matched-pairs signed rank test. All tests were two-tailed and a P value < .05 was considered statistically significant. Kaplan–Meier curves were generated using GraphPad Prism Software. Microsoft Excel (version 14.5.2; Microsoft, Redmond, WA) and GraphPad Prism software (version 6.0; GraphPad Software, Inc., La Jolla, CA) were used.
Results
Patient characteristics
A total of 30 patients (20 males, 10 females) with autoimmune cytopenias intolerant to or having failed other therapies, including corticosteroids, mycophenolate mofetil (MMF), IVIgG, and rituximab, were included and treated with sirolimus (supplemental Table 2). Underlying diagnosis and type of cytopenia and/or organomegaly, and demographic information are shown in Tables 1 and 2 (divided by ALPS vs non-ALPS diagnoses). Of the 30 patients, 12 were diagnosed with ALPS (6 with constitutional FAS [ie, ALPS-FAS] mutations, 3 with somatic FAS mutations [ALPS-sFAS], and 3 with ALPS-U), 8 with Evans syndrome (ES), 2 with common variable immunodeficiency (CVID), 2 with systemic lupus erythematosus (SLE), and 6 with single-lineage cytopenias, including immune thrombocytopenia (ITP) and AIHA (Table 1). The median age of all patients at the time of diagnosis was 5 years (range, 5 months to 19 years) and at the start of sirolimus was 11 years (range, 1.8 to 21 years).
Baseline characteristics, prior treatment outcomes, and sirolimus response in ALPS patients.
| . | . | . | . | Response . | . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 3 mo . | 6 mo . | 12 mo . | . | |||||||
| Disorder . | Lineage cytopenia . | Lymphoproliferative disease . | Prior therapy . | Heme . | Lymphoproliferation . | Heme . | Lymphoproliferation . | Heme . | Lymphoproliferation . | Time on sirolimus . |
| ALPS-FAS | A+N+T | Massive LAD splenomegaly | Corticosteroid (RT) | CR | CR | CR | CR | CR | CR | 8 y |
| IVIgG (PR) | ||||||||||
| MMF (NR) | ||||||||||
| Rituximab (RT) | ||||||||||
| ALPS-FAS | T+N | Massive LAD splenomegaly | Corticosteroid (RT) | CR | CR* | CR | CR* | CR | CR* | 6 y |
| MMF (NR) | ||||||||||
| ALPS-FAS | A+N+T | Massive LAD splenomegaly | Corticosteroid (RT) | CR* | CR* | CR* | CR* | CR* | CR* | 4.5 y |
| IVIgG (NR) | ||||||||||
| MMF (PR) | ||||||||||
| Rituximab (VTR) | ||||||||||
| ALPS-FAS | A+N+T | Massive LAD splenomegaly | Corticosteroid (RT) | CR | CR | CR | CR | CR | CR | 3 y |
| IVIgG (NR) | ||||||||||
| MMF (PR) | ||||||||||
| Rituximab (PR) | ||||||||||
| ALPS-FAS | A+N+T | Marked LAD splenomegaly | Corticosteroid (NR) | CR* | CR* | CR* | CR* | CR* | CR* | 2 y |
| IVIgG (NR) | ||||||||||
| G-CSF (NR) | ||||||||||
| ALPS-FAS | A+T | Marked splenomegaly | Corticosteroid (RT) | PR | CR | PR | CR | CR | CR | 1.5 y |
| IVIgG (RT) | ||||||||||
| ALPS-sFAS | A+N+T | Massive LAD and splenomegaly | Corticosteroids (RT) | CR | CR | CR | CR | CR | CR | 4.5 y |
| MMF (PR) | ||||||||||
| VCR (NR) | ||||||||||
| Pyrimethamine (NR) | ||||||||||
| ALPS-sFAS | A+N+T | Mild LAD and splenomegaly | Corticosteroid (NR) | CR | CR | CR | CR | CR | CR | 4 y |
| IVIgG (NR) | ||||||||||
| MMF (NR) | ||||||||||
| G-CSF (NR) | ||||||||||
| ALPS-sFAS | A+N+T | Massive LAD and splenomegaly | Corticosteroid (RT) | CR | CR* | CR | CR | CR | CR | 4 y |
| Depakote (PR) | ||||||||||
| ALPS-U | A+N+T | Massive LAD and splenomegaly | Corticosteroid (RT) | CR | CR | CR | CR | CR | CR | 3 y |
| IVIgG (NR) | ||||||||||
| MMF (PR) | ||||||||||
| 6-MP (NR) | ||||||||||
| Methotrexate (NR) | ||||||||||
| ALPS-U | A+N+T | Massive LAD and splenomegaly | Corticosteroid (NR) | CR | CR | CR | CR | CR | CR | 3 y |
| IVIgG (NR) | ||||||||||
| ALPS-U | A+N+T | Lymphadenopathy | Corticosteroid (RT) | CR | CR | CR | CR | CR | CR | 3 y |
| IVIgG (PR) | ||||||||||
| . | . | . | . | Response . | . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 3 mo . | 6 mo . | 12 mo . | . | |||||||
| Disorder . | Lineage cytopenia . | Lymphoproliferative disease . | Prior therapy . | Heme . | Lymphoproliferation . | Heme . | Lymphoproliferation . | Heme . | Lymphoproliferation . | Time on sirolimus . |
| ALPS-FAS | A+N+T | Massive LAD splenomegaly | Corticosteroid (RT) | CR | CR | CR | CR | CR | CR | 8 y |
| IVIgG (PR) | ||||||||||
| MMF (NR) | ||||||||||
| Rituximab (RT) | ||||||||||
| ALPS-FAS | T+N | Massive LAD splenomegaly | Corticosteroid (RT) | CR | CR* | CR | CR* | CR | CR* | 6 y |
| MMF (NR) | ||||||||||
| ALPS-FAS | A+N+T | Massive LAD splenomegaly | Corticosteroid (RT) | CR* | CR* | CR* | CR* | CR* | CR* | 4.5 y |
| IVIgG (NR) | ||||||||||
| MMF (PR) | ||||||||||
| Rituximab (VTR) | ||||||||||
| ALPS-FAS | A+N+T | Massive LAD splenomegaly | Corticosteroid (RT) | CR | CR | CR | CR | CR | CR | 3 y |
| IVIgG (NR) | ||||||||||
| MMF (PR) | ||||||||||
| Rituximab (PR) | ||||||||||
| ALPS-FAS | A+N+T | Marked LAD splenomegaly | Corticosteroid (NR) | CR* | CR* | CR* | CR* | CR* | CR* | 2 y |
| IVIgG (NR) | ||||||||||
| G-CSF (NR) | ||||||||||
| ALPS-FAS | A+T | Marked splenomegaly | Corticosteroid (RT) | PR | CR | PR | CR | CR | CR | 1.5 y |
| IVIgG (RT) | ||||||||||
| ALPS-sFAS | A+N+T | Massive LAD and splenomegaly | Corticosteroids (RT) | CR | CR | CR | CR | CR | CR | 4.5 y |
| MMF (PR) | ||||||||||
| VCR (NR) | ||||||||||
| Pyrimethamine (NR) | ||||||||||
| ALPS-sFAS | A+N+T | Mild LAD and splenomegaly | Corticosteroid (NR) | CR | CR | CR | CR | CR | CR | 4 y |
| IVIgG (NR) | ||||||||||
| MMF (NR) | ||||||||||
| G-CSF (NR) | ||||||||||
| ALPS-sFAS | A+N+T | Massive LAD and splenomegaly | Corticosteroid (RT) | CR | CR* | CR | CR | CR | CR | 4 y |
| Depakote (PR) | ||||||||||
| ALPS-U | A+N+T | Massive LAD and splenomegaly | Corticosteroid (RT) | CR | CR | CR | CR | CR | CR | 3 y |
| IVIgG (NR) | ||||||||||
| MMF (PR) | ||||||||||
| 6-MP (NR) | ||||||||||
| Methotrexate (NR) | ||||||||||
| ALPS-U | A+N+T | Massive LAD and splenomegaly | Corticosteroid (NR) | CR | CR | CR | CR | CR | CR | 3 y |
| IVIgG (NR) | ||||||||||
| ALPS-U | A+N+T | Lymphadenopathy | Corticosteroid (RT) | CR | CR | CR | CR | CR | CR | 3 y |
| IVIgG (PR) | ||||||||||
A, anemia; G-CSF, granulocyte colony-stimulating factor; LAD, lymphadenopathy; N, neutropenia; 6-MP, mercaptopurine; T, thrombocytopenia; VCR, vincristine; VTR, very transient response. Prior therapy responses are listed in parentheses: CR, responds but toxicity (RT), PR, PR but relapse (PR-R), and no response (NR).
Designate the patients who remain in a durable CR but have occasional, mild, transient disease flares, manifested as dropping blood counts or the development of lymphadenopathy with viral illness. These “flares” are mild and do not require the use of additional immune suppression, as they self-resolve following the resolution of the infection
Non-ALPS patient baseline characteristics, prior treatment outcomes, and sirolimus response.
| . | . | . | . | Response . | . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 3 mo . | 6 mo . | 12 mo . | . | |||||||
| Disorder . | Lineage cytopenia . | Lymphoproliferative disease . | Prior therapy . | Heme . | Lymphoproliferation . | Heme . | Lymphoproliferation . | Heme . | Lymphoproliferation . | Time on sirolimus . |
| ES | A+N+T | None | Corticosteroids (PR) | PR | N/A | NR | NR | 15 wk | ||
| IVIgG (NR) | ||||||||||
| MMF (PR) | ||||||||||
| Rituximab (NR) | ||||||||||
| ES | A+N+T | Marked LAD and splenomegaly | Corticosteroids (PR) | PR | PR | Off study | Off study | 5 mo | ||
| IVIgG (NR) | ||||||||||
| MMF (NR) | ||||||||||
| Rituximab (NR) | ||||||||||
| ES | A+N+T | Mild splenomegaly | Corticosteroids (PR) | CR | CR | CR | CR | CR | CR | 2 y |
| IVIgG (NR) | ||||||||||
| MMF (NR) | ||||||||||
| Rituximab (allergy) | ||||||||||
| ES | A+N+T | Mild splenomegaly | Corticosteroids (NR) | CR | CR | CR | CR | CR | CR | 2 y |
| IVIgG (NR) | ||||||||||
| MMF (PR) | ||||||||||
| ES | N+T | None | Corticosteroids (RT) | NR | Off study | Off study | N/A | |||
| ES | A+N+T | Mild LAD and splenomegaly | Corticosteroids (RT) | NR | Off study | Off study | N/A | |||
| G-CSF (CR) | ||||||||||
| ES | N+T | None | Corticosteroids (RT) | CR | N/A | CR | CR | 1 y | ||
| IVIgG (PR) | ||||||||||
| ES | N+T | Mild splenomegaly, marked cervical LAD | Corticosteroids (RT) | PR | CR | PR | CR | CR | CR | 1 y |
| IVIgG (NR) | ||||||||||
| CVID | A+N+T | None | Corticosteroid (RT) | CR | N/A | CR | CR | 4.5 y | ||
| IVIgG (NR) | ||||||||||
| Rituximab (NR) | ||||||||||
| CVID | N+T | None | Corticosteroids (RT) | CR | N/A | CR | CR | 3.5 y | ||
| IVIgG (NR) | ||||||||||
| Rituximab (NR) | ||||||||||
| SLE | N+T | None | Corticosteroids (NR) | CR | N/A | CR | CR | 3 y | ||
| IVIgG (NR) | ||||||||||
| Rituximab (NR) | ||||||||||
| SLE | A+N+T | None | Corticosteroids (RT) | PR | N/A | CR | CR | 2.5 y | ||
| IVIgG (NR) | ||||||||||
| Rituximab (NR) | ||||||||||
| ITP | T | None | Corticosteroids (NR) | NR | Off study | Off study | 1 mo | |||
| IVIgG (NR) | ||||||||||
| Splenectomy | ||||||||||
| ITP | T | None | Corticosteroids (NR) | PR | N/A | PR | N/A | Off study | 6 mo | |
| IVIgG (NR) | ||||||||||
| MMF (NR) | ||||||||||
| Rituximab (NR) | ||||||||||
| 6-MP (NR) | ||||||||||
| Hydroxycholoroquine (NR) | ||||||||||
| ITP | T | None | Corticosteroids (NR) | NR | Off study | Off study | 2 mo | |||
| IVIgG (NR) | ||||||||||
| Rituximab (NR) | ||||||||||
| Rh(D) IVIG (NR) | ||||||||||
| Romiplostim (NR) | ||||||||||
| ITP | T | None | Corticosteroids (NR) | NR | Off study | Off study | 3 mo | |||
| IVIgG (NR) | ||||||||||
| Rituximab (NR) | ||||||||||
| Rh(D) IVIgG (NR) | ||||||||||
| Romiplostim (NR) | ||||||||||
| AIHA | A | None | Corticosteroids (RT) | PR | N/A | PR | N/A | PR | N/A | Intermittent 4 y |
| IVIgG (NR) | ||||||||||
| MMF (NR) | ||||||||||
| Rituximab (NR) | ||||||||||
| 6-MP (NR) | ||||||||||
| Splenectomy | ||||||||||
| AIHA | A | Marked LAD and splenomegaly | Corticosteroids (RT) | PR | CR | CR | CR | CR | CR | 1 y |
| IVIgG (NR) | ||||||||||
| MMF (NR) | ||||||||||
| Rituximab (NR) | ||||||||||
| . | . | . | . | Response . | . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 3 mo . | 6 mo . | 12 mo . | . | |||||||
| Disorder . | Lineage cytopenia . | Lymphoproliferative disease . | Prior therapy . | Heme . | Lymphoproliferation . | Heme . | Lymphoproliferation . | Heme . | Lymphoproliferation . | Time on sirolimus . |
| ES | A+N+T | None | Corticosteroids (PR) | PR | N/A | NR | NR | 15 wk | ||
| IVIgG (NR) | ||||||||||
| MMF (PR) | ||||||||||
| Rituximab (NR) | ||||||||||
| ES | A+N+T | Marked LAD and splenomegaly | Corticosteroids (PR) | PR | PR | Off study | Off study | 5 mo | ||
| IVIgG (NR) | ||||||||||
| MMF (NR) | ||||||||||
| Rituximab (NR) | ||||||||||
| ES | A+N+T | Mild splenomegaly | Corticosteroids (PR) | CR | CR | CR | CR | CR | CR | 2 y |
| IVIgG (NR) | ||||||||||
| MMF (NR) | ||||||||||
| Rituximab (allergy) | ||||||||||
| ES | A+N+T | Mild splenomegaly | Corticosteroids (NR) | CR | CR | CR | CR | CR | CR | 2 y |
| IVIgG (NR) | ||||||||||
| MMF (PR) | ||||||||||
| ES | N+T | None | Corticosteroids (RT) | NR | Off study | Off study | N/A | |||
| ES | A+N+T | Mild LAD and splenomegaly | Corticosteroids (RT) | NR | Off study | Off study | N/A | |||
| G-CSF (CR) | ||||||||||
| ES | N+T | None | Corticosteroids (RT) | CR | N/A | CR | CR | 1 y | ||
| IVIgG (PR) | ||||||||||
| ES | N+T | Mild splenomegaly, marked cervical LAD | Corticosteroids (RT) | PR | CR | PR | CR | CR | CR | 1 y |
| IVIgG (NR) | ||||||||||
| CVID | A+N+T | None | Corticosteroid (RT) | CR | N/A | CR | CR | 4.5 y | ||
| IVIgG (NR) | ||||||||||
| Rituximab (NR) | ||||||||||
| CVID | N+T | None | Corticosteroids (RT) | CR | N/A | CR | CR | 3.5 y | ||
| IVIgG (NR) | ||||||||||
| Rituximab (NR) | ||||||||||
| SLE | N+T | None | Corticosteroids (NR) | CR | N/A | CR | CR | 3 y | ||
| IVIgG (NR) | ||||||||||
| Rituximab (NR) | ||||||||||
| SLE | A+N+T | None | Corticosteroids (RT) | PR | N/A | CR | CR | 2.5 y | ||
| IVIgG (NR) | ||||||||||
| Rituximab (NR) | ||||||||||
| ITP | T | None | Corticosteroids (NR) | NR | Off study | Off study | 1 mo | |||
| IVIgG (NR) | ||||||||||
| Splenectomy | ||||||||||
| ITP | T | None | Corticosteroids (NR) | PR | N/A | PR | N/A | Off study | 6 mo | |
| IVIgG (NR) | ||||||||||
| MMF (NR) | ||||||||||
| Rituximab (NR) | ||||||||||
| 6-MP (NR) | ||||||||||
| Hydroxycholoroquine (NR) | ||||||||||
| ITP | T | None | Corticosteroids (NR) | NR | Off study | Off study | 2 mo | |||
| IVIgG (NR) | ||||||||||
| Rituximab (NR) | ||||||||||
| Rh(D) IVIG (NR) | ||||||||||
| Romiplostim (NR) | ||||||||||
| ITP | T | None | Corticosteroids (NR) | NR | Off study | Off study | 3 mo | |||
| IVIgG (NR) | ||||||||||
| Rituximab (NR) | ||||||||||
| Rh(D) IVIgG (NR) | ||||||||||
| Romiplostim (NR) | ||||||||||
| AIHA | A | None | Corticosteroids (RT) | PR | N/A | PR | N/A | PR | N/A | Intermittent 4 y |
| IVIgG (NR) | ||||||||||
| MMF (NR) | ||||||||||
| Rituximab (NR) | ||||||||||
| 6-MP (NR) | ||||||||||
| Splenectomy | ||||||||||
| AIHA | A | Marked LAD and splenomegaly | Corticosteroids (RT) | PR | CR | CR | CR | CR | CR | 1 y |
| IVIgG (NR) | ||||||||||
| MMF (NR) | ||||||||||
| Rituximab (NR) | ||||||||||
N/A, not applicable; other abbreviations are explained in Table 1.
The main indications for sirolimus treatment in all subjects were steroid intolerance and/or the inability to wean off steroids or other immunosuppressants (Tables 1 and 2). All of the patients diagnosed with ALPS were previously treated with corticosteroids. Nine of the 12 patients demonstrated a response to steroids but were unable to maintain the response with weans. The remaining 3 had either a poor or no response (NR) to steroids. Other immunosuppressant agents previously trialed included MMF, rituximab, and IVIgG prior to the start of sirolimus (Table 1). Seven of the 9 patients had NR to IVIgG, 1 of 9 had a transient response but would relapse quickly, whereas 1 had an improvement only in thrombocytopenia. MMF was trialed in 7 of the 12 patients: 3 had NR, whereas the remainder had only a partial response (PR) with a mild improvement in blood counts and no improvement in lymphoproliferation. Supplemental Table 3 reviews the experience of patients treated with MMF in more detail. Two of 4 partial responders required intermittent corticosteroids and even in combination, only had a PR with low but stable blood counts. Four of the 12 patients were treated with rituximab, with only 2 demonstrating a transient response (lasting <3 months) and the other 2 with PR or NR. Other agents trialed included sulfadoxine/pyrimethamine (Fansidar), filgrastim (G-CSF), divalproex sodium (depakote), methotrexate, and mercaptopurine (6-MP). In spite of some anecdotal evidence for these drugs in refractory ALPS, the subjects treated with these drugs prior to sirolimus had NR.
Subjects with multi- and single-lineage cytopenias with or without lymphoproliferative disease not diagnosed with ALPS were similarly trialed with several immunomodulating agents, including steroids, MMF, IVIgG, and rituximab (Table 2). Steroids were the most successful in leading to a response in 9 of 18 subjects; however, as was seen in ALPS subjects, steroids were unable to be weaned. Eleven of 16 subjects treated with IVIgG had NR, poor in 4 subjects, and only 1 subject transiently responded to IVIgG. The response to rituximab was somewhat better, with a CR in 6 of 12, 1 allergic reaction, and 5 NR. However, the CRs were all short-lived. Three subjects relapsed in less than 6 months and the other 3 prior to 1 year. The responses to MMF however were very poor, resulting in an NR (N = 5 of 7) or a PR (N = 2 of 7) (supplemental Table 3).
Pharmacokinetics
After the start of sirolimus, drug troughs were monitored twice weekly until they reached a steady state. All of the patients with ALPS achieved a goal trough level by the first measurement, ranging between 4.6 and 20 ng/mL. Of the other subjects, 4 achieved a suboptimal trough level (range, 2.9 to 4.6 ng/mL) and the remaining patients’ first trough levels ranged between 5.2 to 13.9 ng/mL. In all subjects, only 1 to 3 dose adjustments were necessary, and were made in an effort to achieve more optimal cytopenia responses. Even with these adjustments, the target serum levels were maintained and did not exceed 15 ng/mL.
Efficacy
In subjects with ALPS, 11 of 12 experienced a hematologic CR within 3 months of starting sirolimus (Figures 1 and 2; Table 1). The remaining patient achieved a PR during the first year but reached a CR by 18 months and remains on treatment. Of note, 3 of the patients are in a durable CR, however experience transient disease flares with viral illness. These flares did not require initiation of additional immune suppression. All of the patients were able to wean off corticosteroids. Of the ALPS group, the majority (11 of 12) was able to wean off the other medications within 1 week to 1 month, whereas the 12th patient was weaned off by 3 months.
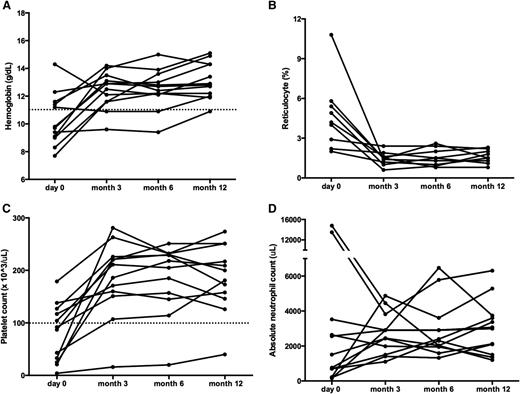
Sirolimus improves multilineage cytopenias in patients with ALPS. Panels depict changes in (A) hemoglobin, (B) reticulocyte count, (C) platelet count, and (D) ANC. All ALPS patients had multilineage cytopenias. Each line represents a different subject’s cell count at each time point measured. The dotted line depicts normal thresholds for hemoglobin and platelets. Although patients may have started below the normal threshold, all but one of the patients reached a CR across all cell lines measured. ANC, absolute neutrophil count.
Sirolimus improves multilineage cytopenias in patients with ALPS. Panels depict changes in (A) hemoglobin, (B) reticulocyte count, (C) platelet count, and (D) ANC. All ALPS patients had multilineage cytopenias. Each line represents a different subject’s cell count at each time point measured. The dotted line depicts normal thresholds for hemoglobin and platelets. Although patients may have started below the normal threshold, all but one of the patients reached a CR across all cell lines measured. ANC, absolute neutrophil count.
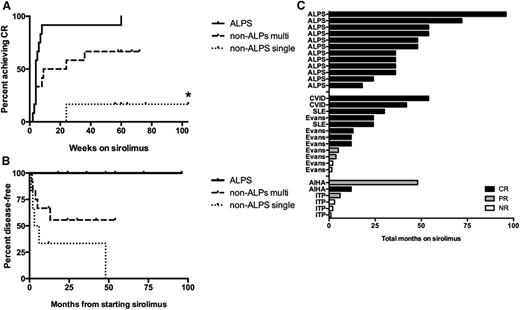
Time to CR, to relapse, and on sirolimus, in ALPS and non-ALPS patients. (A) Overall time to achieve a CR on sirolimus in patients with ALPS compared with non-ALPS patients separated by multilineage vs single-lineage autoimmune cytopenias. For patients whose best response was PR or NR, the curve represents time on sirolimus plus 1 year of observation after discontinuation. The time to CR was not significantly different in ALPS (median, 4 weeks; range, 2 to 60) compared with non-ALPS patients with multilineage cytopenias (median, 9 weeks; range, 2 to 260) (P = .135). In contrast, the time to CR was significantly different in ALPS compared with non-ALPS patients with single-lineage cytopenias (P = .0065). Asterisk (*) represents 1 patient with non-ALPS single-lineage disease who achieved a PR and remained on drug for 260 weeks. (B) Relapse incidence in ALPS and non-ALPS patients with multilineage and single-lineage cytopenias, for all patients enrolled. Events include patients who achieved a CR and then relapsed, and patients who discontinued sirolimus for lack of efficacy. Most of the ALPS patients remained disease-free while on sirolimus, including 1 patient who stopped sirolimus after 3 years but maintained a durable CR. The median time to an event in non-ALPS patients was 13 months. Of note, 2 non-ALPS patients with multilineage cytopenias electively stopped sirolimus, had a transient drop in counts, and then were re-challenged with sirolimus, reverting back to a CR. These patients were not counted as having relapsed. (C) Histogram demonstrating the total time on sirolimus separated by ALPS, non-ALPS multilineage vs non-ALPS–single lineage. The different colors reflect whether they achieved a CR (black), PR (gray), or NR (white).
Time to CR, to relapse, and on sirolimus, in ALPS and non-ALPS patients. (A) Overall time to achieve a CR on sirolimus in patients with ALPS compared with non-ALPS patients separated by multilineage vs single-lineage autoimmune cytopenias. For patients whose best response was PR or NR, the curve represents time on sirolimus plus 1 year of observation after discontinuation. The time to CR was not significantly different in ALPS (median, 4 weeks; range, 2 to 60) compared with non-ALPS patients with multilineage cytopenias (median, 9 weeks; range, 2 to 260) (P = .135). In contrast, the time to CR was significantly different in ALPS compared with non-ALPS patients with single-lineage cytopenias (P = .0065). Asterisk (*) represents 1 patient with non-ALPS single-lineage disease who achieved a PR and remained on drug for 260 weeks. (B) Relapse incidence in ALPS and non-ALPS patients with multilineage and single-lineage cytopenias, for all patients enrolled. Events include patients who achieved a CR and then relapsed, and patients who discontinued sirolimus for lack of efficacy. Most of the ALPS patients remained disease-free while on sirolimus, including 1 patient who stopped sirolimus after 3 years but maintained a durable CR. The median time to an event in non-ALPS patients was 13 months. Of note, 2 non-ALPS patients with multilineage cytopenias electively stopped sirolimus, had a transient drop in counts, and then were re-challenged with sirolimus, reverting back to a CR. These patients were not counted as having relapsed. (C) Histogram demonstrating the total time on sirolimus separated by ALPS, non-ALPS multilineage vs non-ALPS–single lineage. The different colors reflect whether they achieved a CR (black), PR (gray), or NR (white).
Similarly, massive lymphadenopathy and splenomegaly resolved in all patients as early as 3 months from the start of sirolimus (Table 1). Of note, the same patients with transient drops in blood counts with viral illness similarly developed concordant lymphadenopathy during viral illness. Because lymphadenopathy is a common consequence of viral illness, it is unknown whether the flares reflected pathologic or normal reactive lymphadenopathy.
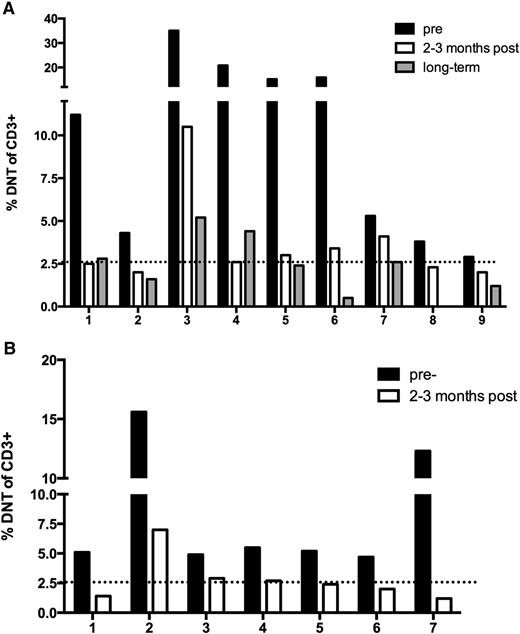
Elevated circulating DNTs, TCRαβ+ CD4−CD8−, are a biologic hallmark and diagnostic criterion for ALPS.11 Small numbers of DNTs circulate in healthy pediatric controls due to a reactive phenomenon and in conditions such as SLE.8,11,12 Therefore, abnormally elevated numbers must exceed 1.5% of total lymphocytes or 2.6% of CD3+ lymphocytes.11 The percentage of DNTs was monitored in the majority of ALPS patients (N = 9) before and after sirolimus. The DNTs dropped to a clinically insignificant percentage in 5 of the patients within 3 months (Figure 3A). The percentage of DNTs decreased 58.9% (range, 20% to 87.5%) within 3 months of initiation of treatment; P = .0039. Long-term follow-up measurements of DNTs (range, 2- to 5-years), demonstrated further resolution with an average decrease of 72.5% (range, 0.5% to 5.2%). One patient’s DNTs were not obtained at the final time point. To further support these findings, additional patients diagnosed with ALPS and treated with sirolimus on a clinical basis were monitored through enrollment on a parallel biology study (Figure 3B). Seven of these subjects also had pre- and post-sirolimus measurement of DNTs, with similar improvements. Of the 7 subjects, 4 had resolution in the DNT percentage ≤2.6% of CD3+ cells. Although not all of the patients had DNT <2.6%, their percent change was just as robust, ranging between 40.8% to 90.2% change, which was statistically significant; P = .0156. As additional validation of the downward trend in DNT percentage after starting sirolimus, absolute DNTs (percentage of DNTs × absolute lymphocyte count [ALC]) were also provided (supplemental Figure 1), demonstrating an almost superimposable trend.
Sirolimus resolves the percentage of DNTs to a normal range in ALPS patients. (A) Patients with ALPS included in the clinical trial. (B) Patients with ALPS included in a biology study, not enrolled in the clinical trial. For both panels, the dotted line represents the normal threshold of circulating DNTs (<2.6%).
Sirolimus resolves the percentage of DNTs to a normal range in ALPS patients. (A) Patients with ALPS included in the clinical trial. (B) Patients with ALPS included in a biology study, not enrolled in the clinical trial. For both panels, the dotted line represents the normal threshold of circulating DNTs (<2.6%).
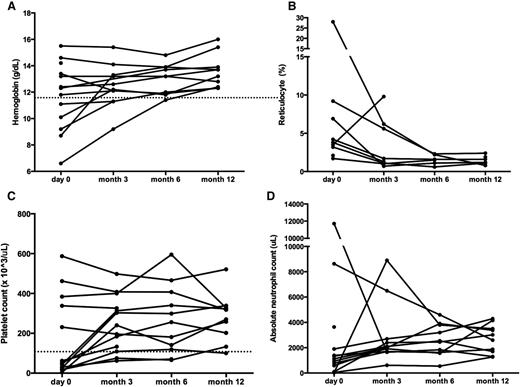
Most of the non-ALPS subjects with multilineage autoimmune cytopenias also responded to sirolimus (Table 2; Figure 4). Three of 8 patients with ES, 2 of 2 CVID patients, and 1 of 2 patients with SLE experienced a CR by 3 months from the start of sirolimus. By 12 months on therapy, 1 patient with ES and the other SLE patient reached a durable CR. Thus, a total of 8 of 12 patients with multilineage autoimmune cytopenias obtained a CR by 1 year on therapy (Figure 2A). One subject stopped sirolimus for toxicity (see “Toxicity/Safety”). Two of the patients with multilineage cytopenias achieved a PR. The remaining 2 of 12 were nonresponders. Importantly, subjects who achieved a CR after starting sirolimus were weaned off other medications within 2 weeks to 1 month.
Sirolimus improves multilineage cytopenias in non-ALPS patients. Panels depict changes in (A) hemoglobin, (B) reticulocyte count, (C) platelet count, and (D) ANC. Each line represents a different patient’s cell count at each time point measured. The dotted line depicts normal thresholds for hemoglobin and platelets. ANC, absolute neutrophil count.
Sirolimus improves multilineage cytopenias in non-ALPS patients. Panels depict changes in (A) hemoglobin, (B) reticulocyte count, (C) platelet count, and (D) ANC. Each line represents a different patient’s cell count at each time point measured. The dotted line depicts normal thresholds for hemoglobin and platelets. ANC, absolute neutrophil count.
The responses in patients with single-lineage autoimmune cytopenias were less robust (Figures 2A and 4). Four children with ITP were treated, of which 3 did not respond, and 1 subject had a PR that lasted ∼6 months before therapy was stopped. Two children with AIHA were treated. One had a CR by 6 months on therapy and was able to wean off other medications within a month. Of note, this patient has a mutation in CASP10, an ALPS-associated gene; however, this subject did not meet the diagnostic criteria for ALPS because DNTs were not elevated at baseline. The other child with AIHA achieved a PR; although this patient had a complete resolution of cytopenias when compliant with sirolimus, likely reflecting adequate serum levels; when the subject discontinued sirolimus (which happened often), his disease would flare.
Many of the patients who achieved a CR have been continued on sirolimus with durable responses (Figure 2B-C). Follow-up of all patients ranged from 0 to 8 years, with a median of 2 years. All of the ALPS patients experienced durable responses and chose to remain on sirolimus beyond the study goal of 6 months (median, 3.5 years; range, 1.5 to 8 years). Further, those patients who achieved a CR were able to wean off all corticosteroids. Only 1 ALPS patient chose to discontinue sirolimus and has not had any exacerbation of the underlying cytopenias.
Of the non-ALPS patients, the length of time on sirolimus was shorter, ranging between 1 to 4.5 years (Figure 2B-C). Not surprisingly, the subjects with multilineage disease (ie, ES, CVID, or SLE) were more likely to remain on study, whereas only one subject with single-lineage disease remained on sirolimus for up to 1 year, after achieving a CR. The remainder came off study between 2 to 6 months mostly due to nonresponse. Of note, the shorter follow-up time partly reflects the timing of the study not open to non-ALPS subjects for the first 2 years.
Toxicity/safety
Overall, sirolimus was well tolerated with few side effects. The presence and/or the severity of toxicity did not correlate with trough levels or the likelihood to achieve a CR. The most common adverse effect of sirolimus was grade 1 to 2 mucositis (N = 10 of 30). This occurred most often within the first 3 months of initiating sirolimus and resolved without therapy modification. Other toxicities included elevated triglycerides and elevated cholesterol (n = 2), that responded to fish oil or atorvastatin. One patient developed hypertension 2 years after starting sirolimus, although this was temporally related to starting a new psychiatric medication. Other side effects included acne (N = 1), sun sensitivity (N = 1), and exacerbation of gastro-esophageal reflux disease (N = 1). One subject with ES achieved a PR after 3 months; however, later developed a headache with associated white matter changes (4 different lesions) noted by brain magnetic resonance imaging. These changes were not consistent with posterior reversible encephalopathy syndrome or posterior multifocal leukoencephalopathy. An extensive workup was negative for an infectious etiology, including JC polyomavirus or demyelination. Sirolimus was discontinued and the headaches resolved within a few days. This subject successfully reached normal blood counts on sirolimus and weaned off all other immune suppressive medications. Nevertheless, based on toxicity, the sirolimus was discontinued prior to determining if the patient could maintain normal counts for 2 months, and this patient was classified as having a PR. The changes were eventually attributed to disease-associated vasculitis, and the lesions noted on magnetic resonance imaging resolved over the next few months with the addition of steroids. This patient was eventually diagnosed with a primary T-cell immune deficiency and underwent hematopoietic stem cell transplantation.
Immune function
Sirolimus is most commonly used to suppress the immune system and prevent rejection after solid organ transplant. Often, sirolimus is used in combination with other immunosuppressive drugs, making it challenging to understand the risk for infection noted in the literature associated with these drugs.13 Therefore, we wanted to ensure that sirolimus monotherapy did not impair the function of normal immune cells.
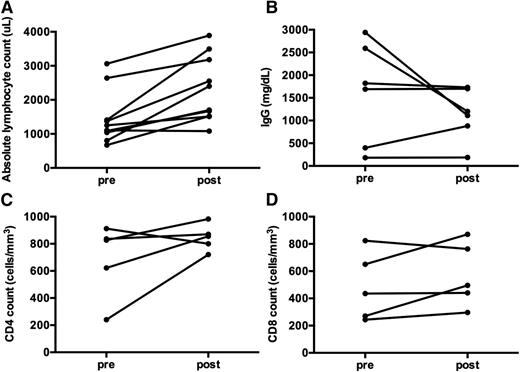
Quantitative and qualitative assessments of immune function were obtained in a subset of patients (Figures 5 and 6). Overall, ALPS patients treated with sirolimus did not have a reduction in B- or T-cell number or function, as assessed by ALC, quantitative IgG, CD4, and CD8 counts, or mitogen stimulation after over 1 year on therapy (Figure 5).
Immune function is not affected by long-term treatment with monotherapy sirolimus in ALPS patients. (A) ALC significantly improved pre- vs post-sirolimus treatment (P = .039). (B) Quantitative IgG was measured in 6 subjects. Prior to starting sirolimus, the IgG was elevated in 3 children, low in 2, and normal in 1. The IgG normalized in 5 of the 6 children with time (P = .5625). One subject had persistent hypogammaglobulinemia and was diagnosed with comorbid CVID prior to starting sirolimus. Interestingly, the other subject with hypogammaglobulinemia developed it while on chronic MMF, requiring chronic IVIgG replacement for many years. After starting sirolimus, the hypogammaglobulinemia improved. The child was subsequently challenged with vaccines and demonstrated normal antibody response, and was able to discontinue IVIgG supplementation. (C) CD4 and (D) CD8 counts were also studied in 5 ALPS patients pre- and post- long-term administration of sirolimus, with no decrease in number (P = .1875 and P = .3125, respectively). Although not depicted here, 5 ALPS patients had mitogen stimulation measured between 2 and 5 years after initiation of sirolimus, with robust responses to phytohemagglutinin A, concanavalin A, and poke weed mitogen. Measurements post-sirolimus treatment varied per subject, between 1 to 5 years of treatment.
Immune function is not affected by long-term treatment with monotherapy sirolimus in ALPS patients. (A) ALC significantly improved pre- vs post-sirolimus treatment (P = .039). (B) Quantitative IgG was measured in 6 subjects. Prior to starting sirolimus, the IgG was elevated in 3 children, low in 2, and normal in 1. The IgG normalized in 5 of the 6 children with time (P = .5625). One subject had persistent hypogammaglobulinemia and was diagnosed with comorbid CVID prior to starting sirolimus. Interestingly, the other subject with hypogammaglobulinemia developed it while on chronic MMF, requiring chronic IVIgG replacement for many years. After starting sirolimus, the hypogammaglobulinemia improved. The child was subsequently challenged with vaccines and demonstrated normal antibody response, and was able to discontinue IVIgG supplementation. (C) CD4 and (D) CD8 counts were also studied in 5 ALPS patients pre- and post- long-term administration of sirolimus, with no decrease in number (P = .1875 and P = .3125, respectively). Although not depicted here, 5 ALPS patients had mitogen stimulation measured between 2 and 5 years after initiation of sirolimus, with robust responses to phytohemagglutinin A, concanavalin A, and poke weed mitogen. Measurements post-sirolimus treatment varied per subject, between 1 to 5 years of treatment.
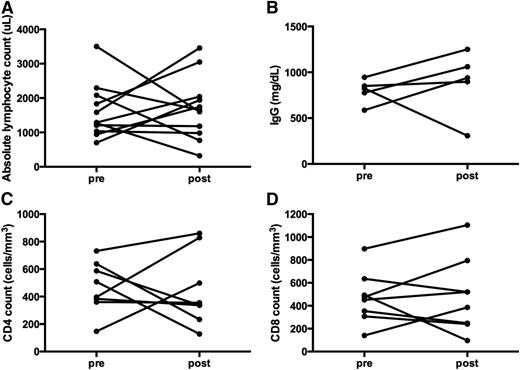
Immune function measured following long-term treatment with monotherapy sirolimus in non-ALPS patients. (A) ALC. Although not statistically significant, 5 of the 12 subjects demonstrated an increase in the ALC, 2 with no change, 2 with decreases but remained normal, and 3 subjects whose ALC dropped to a more moderate lymphopenia range (post-sirolimus ALC range, 320 to 3456 μL; P > .999). (B) IgG quantification; it remained in a normal range for most, and were unchanged before and after the use of sirolimus (P = .8438). (C) CD4 and (D) CD8 counts were monitored pre- and post- long-term administration of sirolimus. CD4 and CD8 counts mostly either remained the same or increased (P = .8438 and P = .6250, respectively). Three subjects had a decrease in the CD4 count; however, only 1 fell to a level <200 μL. That same patient also demonstrated a drop in the CD8 count to a low level (from 492 to 96 μL), after 3 months of sirolimus treatment. Also not depicted here, the response to mitogens was available on 3 subjects: 1 remained normal at 1 year, whereas 2 were reduced at 1 and 4 years. Measurements post-sirolimus treatment varied per subject, between 3 months to 4 years of treatment.
Immune function measured following long-term treatment with monotherapy sirolimus in non-ALPS patients. (A) ALC. Although not statistically significant, 5 of the 12 subjects demonstrated an increase in the ALC, 2 with no change, 2 with decreases but remained normal, and 3 subjects whose ALC dropped to a more moderate lymphopenia range (post-sirolimus ALC range, 320 to 3456 μL; P > .999). (B) IgG quantification; it remained in a normal range for most, and were unchanged before and after the use of sirolimus (P = .8438). (C) CD4 and (D) CD8 counts were monitored pre- and post- long-term administration of sirolimus. CD4 and CD8 counts mostly either remained the same or increased (P = .8438 and P = .6250, respectively). Three subjects had a decrease in the CD4 count; however, only 1 fell to a level <200 μL. That same patient also demonstrated a drop in the CD8 count to a low level (from 492 to 96 μL), after 3 months of sirolimus treatment. Also not depicted here, the response to mitogens was available on 3 subjects: 1 remained normal at 1 year, whereas 2 were reduced at 1 and 4 years. Measurements post-sirolimus treatment varied per subject, between 3 months to 4 years of treatment.
Immune function was also measured in 12 subjects with multilineage cytopenias without the diagnosis of ALPS, between 3 months to 4 years after the start of sirolimus (Figure 6). Unlike the patients with ALPS, the responses were more heterogeneous: some patients had reductions in B- and T-cell number and function, whereas others did not.
Discussion
The management of patients with chronic refractory autoimmune cytopenias is complex. Overall, goals focus on treatment of the primary disease manifestations because a cure is presently not possible outside of hematopoietic stem cell transplantation. Typically, these patients are treated with nonspecific immune suppression, which is often ineffective and toxic. For patients with secondary autoimmune cytopenias, the primary goal is often directed at treating the underlying cause of the autoimmunity. Patients with autoimmune cytopenias as a consequence of SLE are typically treated with medications active in SLE, whereas patients with CVID are often treated with higher doses of IVIgG. Nevertheless, patients with these conditions often fail traditional and “disease-directed” therapies, often because little is known regarding underlying disease biology and targeted medications do not exist. Frequently, agents that are studied in a given disease are chosen based on provider comfort in the specialty; for example, cyclophosphamide and methotrexate are commonly used in patients with a large number of rheumatologic conditions despite the biologic rationale that these drugs would be inferior to more targeted therapies.
ALPS patients are typically treated with steroids and IVIgG, and many patients often respond well to steroids. However, because ALPS is a chronic disease, many patients will need chronic steroid treatment that is associated with significant long-term morbidity, especially in terms of infection and bone health.1 Alternatively, although IVIgG is often used in ALPS, many of these patients do not respond, except in patients with single-lineage autoimmune thrombocytopenia.1,11 Rituximab and splenectomy have additional risks in ALPS patients that should be considered before using such therapies. Rituximab can lead to prolonged, clinically significant hypogammaglobulinemia and eventual CVID when used in ALPS patients.1,4 Splenectomy is not without risk in any population with the development of post-splenectomy sepsis, even with antibiotic prophylaxis and vaccination.8,10 The risk has been noted to be even higher in patients with ALPS because they lack circulating CD27+ memory B-lymphocyte populations.2,8,11 Thus, in our opinion, splenectomy and rituximab should be discouraged unless they are the only remaining measure to control chronic refractory, life-threatening cytopenias.8
The best-studied steroid-sparing agent used in children with ALPS is MMF. Although MMF has been demonstrated to lead to measurable improvements in autoimmune disease and is well tolerated, MMF does not induce lymphocyte death nor has it been demonstrated to have any effect on lymphoproliferative disease or the reduction of DNTs.1,5 As a result, some ALPS patients treated with MMF alone may have PRs and/or eventual relapse. Moreover, the subjects in our series either failed (N = 3) or had a PR (N = 4) to MMF, prompting a need for alternative, steroid-sparing therapy. We recommend considering MMF in patients with mild to moderate autoimmune cytopenias that do not have clinically significant lymphoproliferation (organ compromise or hypersplenism). For patients who fail MMF, have moderate to severe autoimmune cytopenias, or have clinically significant lymphoproliferation, we recommend sirolimus.
We have previously described a retrospective cohort demonstrating the initial evidence of successful use of sirolimus in ameliorating autoimmune disease and lymphoproliferation in patients with ALPS.5 Recently Miano et al published a single institutional series treating patients with refractory autoimmune cytopenias with sirolimus.14 In this study of patients with chronic/refractory autoimmune cytopenias, sirolimus was used in 16 patients as a third-line treatment, of which 75% responded; whereas 67% of those patients had previously failed MMF. Of note, however, no ALPS patients received sirolimus in that study.
This is the first prospective multicenter clinical trial evaluating the safety and efficacy of sirolimus for patients with refractory autoimmune multilineage cytopenias. We found that sirolimus is a safe and effective, steroid-sparing monotherapy for refractory autoimmune cytopenias. Sirolimus not only leads to improvements in cytopenias, it also leads to improvement in lymphoproliferation, as the enlarged lymph nodes and spleen shrink significantly with the reduction of the signature DNT cell numbers. In patients with ALPS, they all achieved a CR for both autoimmune cytopenias and manifestations of lymphoproliferative disease. Further, DNTs in the ALPS subjects dropped to a normal range (≤2.6%). We have also guided clinicians in the treatment of over 30 additional children with ALPS from 18 different countries on 6 continents, with sirolimus. As these are anecdotal, as we cannot report efficacy or safety data; however, we can report that sirolimus is a feasible option for children worldwide.
Based on the results seen in patients with ALPS, we also treated patients with non-ALPS autoimmune cytopenias. In these patients with multilineage autoimmune cytopenias, including ES, CVID, and SLE, there was a similar dramatic response to sirolimus noted with a CR in 67% (8/12) of patients. Only 6 of 12 non-ALPS patients (with multilineage cytopenias) manifested lymphoproliferative disease, including mild to moderate lymphadenopathy and/or splenomegaly, with the majority (67%, 4/6) having achieved a CR in lymphoproliferation, 1 patient with a PR (17%, 1/6), and 1 patient was a nonresponder (16%, 1/6).
Drugs such as MMF, sirolimus, or tacrolimus are often used as part of a multi-agent immunosuppressant backbone following organ or marrow transplant, or as a part of a chemotherapy regimen for lymphoid malignancies.1 The common concern for excess risk for infection likely reflects these agents when used in combination and has remained unfounded with monotherapy. Correspondingly in our study, we demonstrate that the immune function of cells was not compromised in most patients on sirolimus, because there were continued robust and appropriate responses to mitogen stimulation, and stable, if not improved, immune cell numbers (ALC, CD4, and CD8 counts; Figures 5 and 6). Our data suggest the importance of measuring immune function in patients with different diseases when treated with novel agents; patient-specific data should guide the use of prophylactic antimicrobials in order to assess risk for infection. Based on our results, we do not recommend pneumocystis, antifungal, or antibacterial prophylaxis while on single-agent sirolimus.
Based on the results of this study, sirolimus led to complete and durable responses in a majority of children with multilineage autoimmune cytopenias. The responses in patients with ALPS were profound, and these results suggest that sirolimus should be considered early in the management of patients who need chronic treatment. Of note, whereas the effect of sirolimus is robust, immune suppressants like sirolimus and MMF take time before achieving their full effects. Thus, patients who present with severe or life-threatening autoimmune cytopenias likely need corticosteroids or IVIgG as bridging therapy because they have a more rapid onset. Similarly, we think sirolimus should be considered in patients with multilineage autoimmune cytopenias not diagnosed with ALPS, although more studies are needed for children with ES, CVID, and SLE or other autoimmune scenarios to demonstrate safety and efficacy. An interesting notion is whether or not sirolimus can be safely stopped after a durable CR is achieved. The 1 ALPS patient who was successfully discontinued from sirolimus and remains in CR was diagnosed with ALPS-U. Further, 1 non-ALPS patient with ES who had a CR for over 1 year also stopped sirolimus but had a significant life-threatening relapse 2 months later. This child did not respond to sirolimus when it was restarted, raising some caution. Therefore, further studies are needed to determine whether discontinuation of sirolimus is possible, although we believe that the side-effect profile of sirolimus is more than safe and acceptable long term, especially in comparison with the other available agents, including corticosteroids.
Interestingly, there are a number of recently described novel mutations described in patients that mirror ALPS, eg, mutations in CTLA4 or PIK3C.15-17 In fact, some patients identified with PIK3C have already been demonstrated to respond to sirolimus in a pilot trial.16 Although we are unable to test our patients for these newly described mutations, their identification support additional rationale for the use of sirolimus, and may help identify those patients more likely to respond based on disease biology.
In conclusion, this prospective trial supports the use of sirolimus in patients with refractory multilineage cytopenias. Sirolimus is an effective, well-tolerated medication that has yielded durable and dramatic responses, and should be considered for refractory patients.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank all participants in this study and their families.
This study was supported by grant funding from Cures within Reach (previously known as the Partnership for Cures Patient Impact Initiative), the United States Immunodeficiency Network through the National Institutes of Health National Institute of Allergy and Infectious Diseases (N01-A1-30070, R56A1091791, and R21A1099301), a Foerderer-Murray Award, the Goldman Philanthropic Partnerships and the Rockefeller Brothers Fund, the Barbara Brodsky Foundation, as well as from generous donations by families cared for at the Children’s Hospital of Philadelphia. The funding sources had no role in data collection, analysis, or interpretation of the data, the writing of the report, or the decision to submit for publication.
Authorship
Contribution: K.L.B. and D.T.T. designed the study, analyzed data, and wrote the manuscript; T.V. and J.C. contributed to data acquisition, study design, and manuscript editing; and K.S.-W., M.P.L., J.J.B., A.E.S., C.S.M., and S.A.G. contributed to study design and manuscript editing.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: David T. Teachey, CTRB3008, CHOP, 34th and Civic Center Blvd, Philadelphia, PA 19104; e-mail: teacheyd@chop.edu.