Chimeric antigen receptor T cells redirected to CD19 (chimeric antigen receptor [CAR19]) show great promise in the clinic to treat refractory CD19+ acute lymphoblastic leukemia (ALL). However, production of autologous CAR19 cells from these patients can be difficult as patients frequently have T-cell dysfunction, due to disease and/or treatment-related effects. In this issue of Blood, Jacoby et al1 addressed this by exploring whether allogeneic donor CAR19 cells could be used to treat ALL-bearing mice using a minor mismatch bone marrow transplant model.
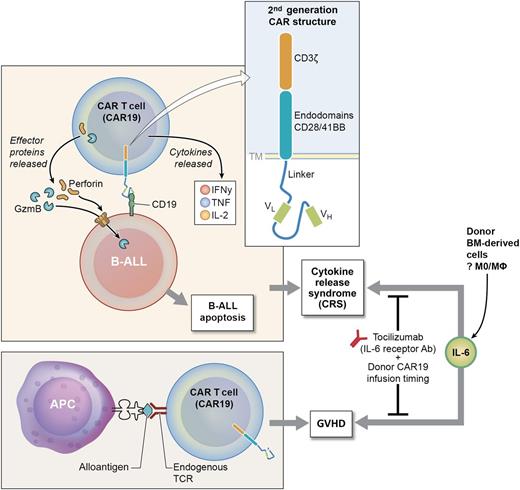
Allogeneic CAR T cells: balancing antitumor vs graft host disease. BM donor CAR T cells directed to CD19 (CAR19) recognize CD19 on B-ALL cells and release effector proteins (perforin and granzyme B) inducing B-ALL apoptosis. Cytokines (in particular IL-6) can be released at high levels, inducing CRS, a serious clinical toxicity. In addition, a subset of donor CAR19 cells (mostly CD4+ CAR19) recognize host alloantigen and induce GVHD. Data from Jacoby et al, and others, indicate both GVHD and CRS can be alleviated by careful timing of the donor CAR19 infusion and using the IL-6R antibody (tocilizimab). APC, antigen-presenting cell; IFNγ, interferon γ; TCR, T-cell receptor. Professional illustration by Ken Probst, XavierStudio.
Allogeneic CAR T cells: balancing antitumor vs graft host disease. BM donor CAR T cells directed to CD19 (CAR19) recognize CD19 on B-ALL cells and release effector proteins (perforin and granzyme B) inducing B-ALL apoptosis. Cytokines (in particular IL-6) can be released at high levels, inducing CRS, a serious clinical toxicity. In addition, a subset of donor CAR19 cells (mostly CD4+ CAR19) recognize host alloantigen and induce GVHD. Data from Jacoby et al, and others, indicate both GVHD and CRS can be alleviated by careful timing of the donor CAR19 infusion and using the IL-6R antibody (tocilizimab). APC, antigen-presenting cell; IFNγ, interferon γ; TCR, T-cell receptor. Professional illustration by Ken Probst, XavierStudio.
In this murine model, allogeneic CAR19 cells were very effective in eradicating ALL; they also induced graft-versus-host disease (GVHD) in host mice. The GVHD only occurred when the recipient mice were leukemia bearing and was caused by CD4+ CAR19 cells. This GVHD response occurred in the context of a proinflammatory microenvironment, as CAR19 cells were given 2 days after bone marrow transplantation (BMT) conditioning therapy. In contrast, when an alternate BMT model with delayed allogeneic CAR19 infusion was used, GVHD was ameliorated. Furthermore, GVHD was abrogated when CAR19 cells were given to leukemia-bearing hosts reconstituted with interleukin (IL)-6−/− donor BM. It is not yet clear which donor BM cells are the critical source of IL-6 in this model (see figure).
These data at first appear to contradict the clinical data reported by the authors2 ; however, the patients who received allogeneic CAR19 received treatment of persistent disease after transplant and donor lymphocyte infusion (DLI) infusion with no or very mild GVHD. The CAR19 cells were given with no chemotherapy or conditioning regimens. Jacoby et al show that the timing of the CAR19 therapy and conditioning therapy is critical for determining clinical outcome. In addition, their model system provides key information regarding the importance of IL-6 biology in clinical response to CAR T-cell therapy. The IL-6R blocking antibody (tocilizumab) has now been used successfully to treat patients with cytokine release syndrome (CRS) following CAR19 therapy3,4 or GVHD.5 The study by Jacoby et al indicates that tocilizumab could be used for autologous CAR19 therapy, as there was no apparent difference in antitumor efficacy, and toxicity may be reduced. In addition, tocilizumab could be used for allogeneic donor-derived CAR19 DLI therapy after BMT.
The CAR T-cell field is going through rapid change, and new CARs are being designed, tested in preclinical models, and rapidly transitioned into the clinic. In parallel with the study by Jacoby et al, alternate strategies include donor-derived CAR T cells where endogenous TCR expression has been silenced.6 This CAR19 product induced a favorable antitumor response, with no GVHD, in 1 patient with refractory ALL. We wait for ongoing clinical data to establish whether the initial clinical outcome is durable and more broadly applicable.
The CAR T-cell field has historically focused on using autologous T cells to derive the therapeutic dose. Human immunology studies and clinical trials in patients with ALL revealed that a preexisting defect in T-cell biology makes the autologous CAR T-cell strategy, at times, fraught with difficulty and can produce a suboptimal product for patients. The allogeneic donor CAR T-cell strategy provides one avenue to address this issue. Clearly, this is associated with a concomitant risk of GVHD. There are 2 distinct approaches to deal with the GVHD issue: either silencing of the endogenous TCR expression (coupled with inducible caspase 9) or infusion of CAR19 cells in the context of a BMT. In the current study, the authors indicate that careful adjustment of the treatment protocol, including judicious use of IL-6R blocking antibody could circumvent GVHD. An alternate approach would be to use either natural killer or nonconventional donor T cells to derive CAR19 cells. Nonconventional T cells have the added advantage of a semirestricted TCR repertoire and reduced risk of GVHD. Natural killer T cells7 and γδ T cells8 have already been successfully transduced to express CAR and showed an antitumor response in vitro and in vivo. In contrast, CAR transduction data and antitumor effect are completely unknown for mucosal-associated invariant T cells.
In conclusion, healthy donor-derived T cells provide a potential twofold benefit to the CAR T-cell field. First, healthy donor T cells are a reliable avenue to making potent antitumor CAR T cells for individual patients. Second, healthy donor T cells also provide a conduit to regular and rapid production of CAR T cell–based products for the more general application of this therapy in the clinic. Thus, normal donor CAR T cells could complement the already spectacular success of the autologous CAR-T cell programs.
Conflict-of-interest disclosure: P.N. has received royalties from Juno Therapeutics.