Abstract
Platelets are anucleate cytoplasmic discs derived from megakaryocytes that circulate in the blood and have major roles in hemostasis, thrombosis, inflammation, and vascular biology. Platelet transfusions are required to prevent the potentially life-threatening complications of severe thrombocytopenia seen in a variety of medical settings including cancer therapy, trauma, and sepsis. Platelets used in the clinic are currently donor-derived which is associated with concerns over sufficient availability, quality, and complications due to immunologic and/or infectious issues. To overcome our dependence on donor-derived platelets for transfusion, efforts have been made to generate in vitro–based platelets. Work in this area has advanced our understanding of the complex processes that megakaryocytes must undergo to generate platelets both in vivo and in vitro. This knowledge has also defined the challenges that must be overcome to bring in vitro–based platelet manufacturing to a clinical reality. This review will focus on our understanding of committed megakaryocytes and platelet release in vivo and in vitro, and how this knowledge can guide the development of in vitro–derived platelets for clinical application.
Introduction
The clinical challenge
Donor-derived platelets are used for the primary purposes of (1) standard transfusions requiring large numbers of quiescent prophylaxis platelets responsive to vascular injury1 and (2) acute (often posttrauma) transfusional needs requiring platelets that are immediately responsive for incorporation into sites of injury.2 These clinical needs for platelet transfusions are extensive and increasing. More than 106 units of donor-derived platelets are administered in the United States each year for bleeding-associated trauma and surgery, chemotherapy/radiation-induced thrombocytopenia, sepsis, and other indications.3 As technologies increase to keep greater numbers of critically ill patients alive and to perform more high-risk medical interventions, requirements for platelet units will continue to increase. However, the number of available platelet donors has not kept pace with rising platelet transfusion requirements, and this discrepancy will likely increase, given the demography of aging in the United States. Compounding these platelet supply challenges are issues with individual units, including variations in platelet number and functionality, room temperature storage requirements that increase the risk of bacterial contamination, up to 5-day expiration dates resulting in discarded units that create wastage, and platelet short half-lives of 1.5 to 3 days following infusion. The generation of an efficient, non-donor-dependent system for platelet production to supplement the donor-derived pool could address many of these concerns.
Overview of megakaryopoiesis and thrombopoiesis
Megakaryopoiesis is the process by which a hematopoietic progenitor cell (HPC) differentiates into a large polyploid megakaryocyte, whereas thrombopoiesis is the process by which platelets are released from megakaryocytes.4 As megakaryocytes mature in the bone marrow, the nucleus becomes multilobed by endomitosis and the cytoplasm undergoes an increase in volume. Important features of the mature megakaryocyte are: (1) the formation of an elaborate demarcation membrane system (DMS), elegantly shown to originate from focal points in the plasma membrane that appear to invaginate between nuclear lobes with expansion contributed by intracellular vesicular membranes5 ; (2) increased numbers of α and dense granules concomitant with cytoplasmic expansion; and (3) a dense tubular network with an open canalicular system created for granule release. The DMS functions as a membrane reservoir for the formation of proplatelets that extend into the bone marrow sinusoids where fragments are released for final platelet processing in the circulation and/or pulmonary vasculature.6-10 High ploidy is not a prerequisite to generate platelets as shown by the ability of cord blood–derived megakaryocytes of 2 to 4N average ploidy and the newly described embryonic diploid proplatelet-forming cells to release platelets.11-14 The recent work on the biogenesis of the DMS suggests a mechanistic cross-talk between endomitosis, cytoplasmic maturation, and platelet formation,5 but the exact mechanism and trigger of proplatelet formation and platelet release are still unknown.
Stem cell–differentiation systems and their challenges
Following the discovery of the central role of the mpl receptor and its ligand thrombopoietin (TPO) in megakaryopoiesis (reviewed in Kaushansky15 ), culture systems for megakaryocytes were quickly developed and the first report of in vitro–generated platelets was published 20 years ago.16 Excellent reviews covering the sources of HPCs used to generate in vitro megakaryocytes (including culture conditions and yields) have been written; the reader is referred to these for details of methodology.17-19 Systems using fetal and adult CD34+ HPC-derived megakaryocytes have advanced our knowledge of megakaryopoiesis and thrombopoiesis, but these cells fall short of being an optimal source for in vitro–derived platelets because of the need of a continuous donor supply, increased apheresis costs due to CD34 selection, and issues of alloreactivity. Human pluripotent stem cells (PSCs), including embryonic stem cells (ESCs) and induced PSCs, offer another platform for generating in vitro–derived megakaryocytes and platelets. The advantages of these cells are that they self-replicate indefinitely in culture, are amenable to genetic manipulation, and provide an unlimited source of megakaryocytes. A major disadvantage with all systems is delivering the yield and quality of platelets necessary for clinical application.
Two major strategies exist in the literature for obtaining platelets from in vitro–derived megakaryocytes. One strategy is to harvest platelets directly from megakaryocytes in vitro or via a platelet manufacturing bioreactor-like system; another strategy is to infuse in vitro–generated megakaryocytes followed by platelets released in vivo. These strategies are described in 2 recent publications that have expressed views for the arrival of in vitro–derived platelets in the clinical setting from optimistic20 to cautiously optimistic.21 The Lanza group20 demonstrated good manufacturing practice feasibility of the stem cell–differentiation systems, which is a step forward in the production line of in vitro–generated platelets. They also demonstrated increased megakaryocyte and platelet yields, enabling platelet function to be analyzed by the traditional method of light transmission aggregometry. Unfortunately, platelet responsiveness was low and the in vivo half-life of these platelets using immunodeficient mice required macrophage depletion by clodronate treatment,22 suggesting suboptimal quality of the infused platelets. Our group used the immunodeficient mouse model without clodronate treatment as a 2-pronged approach: (1) for generating platelets following infusion of in vitro–generated megakaryocytes and (2) as an indicator of the quality and/or purity of the infusate containing the megakaryocyte populations. This system is based on reports that the pulmonary vasculature, as the first capillary bed encountered by megakaryocytes and/or cytoplasmic fragments leaving the bone marrow, may provide a unique environment for platelet shedding.10,23 The feasibility and safety of megakaryocyte infusion in humans has been reported,24,25 with potential limitations including use of this approach in patients with (or at risk of) pulmonary disease and time delays in achieving a maximal rise in platelet counts. By using this approach in mice26 and comparing the yield and half-life of platelets released from megakaryocytes generated from adult, fetal liver, and PSC-derived progenitors to donor-derived platelets, 2 pools of human platelets were detected. One pool, comparable in size, yield, half-life, and function to donor-derived platelets, was generated from the infused megakaryocytes after a few hours delay. Another pool of platelet-like particles (PLPs) present in the megakaryocyte infusate was detected immediately upon infusion that differed in size, yield, half-life, and function to donor-derived platelets. This PLP half-life was markedly improved following clodronate treatment of the mice. These data suggested that stem cell–differentiation systems generate mature megakaryocytes that release platelets comparable in quality to donor-derived platelets, but these in vitro culture systems also generate damaged megakaryocytes that release PLPs. The injuries that occur to cultured megakaryocytes and released platelets are major challenges that require better definition; strategies need to be developed to avoid or eliminate them. On average, ∼10 to 20 human megakaryocytes/starting stem or progenitor cells are generated in vitro, but of that total number only ∼10% to 30% show characteristics of generating platelets as evidenced by proplatelet formation (PPF).9,27 This number can be increased when cells are cultured in the presence of extracellular matrix proteins, but final platelet yield/megakaryocyte numbers of <50 remain far below the estimated 103 platelets released from bone marrow–derived megakaryocytes. Each apheresis donor unit contains ∼3 × 1011 platelets making it logistically difficult and prohibitively expensive to generate such numbers without major improvements to the current stem cell–differentiation systems.
This review focuses on the challenges of improving megakaryocyte and platelet yield in the current stem cell-differentiation systems. To overcome these challenges, recent findings in the areas of apoptosis, receptor sialylation and TPO regulation, self-renewing megakaryocyte progenitors, and platelet manufacturing systems will be discussed.
Overcoming the challenges
Megakaryocyte in vitro injuries
Apoptosis: intrinsic pathway.
Apoptosis in platelet generation has been previously studied; the reader is referred to an excellent review covering the historical perspective of this topic.28 A renewed interest in the role of the apoptotic pathway in megakaryopoiesis and platelet generation came with the success of BH3 mimetics that target the intrinsic apoptotic pathway and are used as antitumor drugs for the treatment of cancer. One drug in particular, ABT-73729 or navitoclax,30 induced thrombocytopenia in patients due to the off-target inhibition of the prosurvival protein Bcl-xL. Recent work has shown that platelet lifespan is dependent on Bcl-xL, which when neutralized by ABT-737 activated apoptosis primarily through the proapoptotic mediator Bak.31 Bak triggers the mitochondrial outer membrane pore, which is an irreversible step to apoptotic death.32 By using knockout mice and BH3 mimetics, elegant studies have shown that bone marrow megakaryocytes must restrain the intrinsic or mitochondrial apoptotic pathway to survive and release platelets. In megakaryocytes, the critical regulators are the Bcl-2 family members, Bcl-xL and Mcl-1,33,34 whereas platelet lifespan is determined solely by Bcl-xL.31,35,36 Mice with a megakaryocyte-specific deletion of Bcl-xL were thrombocytopenic with an abundance of phosphatidylserine-positive microparticles in platelet-rich plasma; decreased numbers of CD41, glycoprotein IX (GPIX), GPIbα, or GPVI-expressing cells; and decreased platelet half-life to 5 hours.37 Megakaryocyte-specific deletion of both Bcl-xL and Mcl-1 predisposed the cells to spontaneous apoptosis in vivo resulting in a preweaning lethality at 21 days of age from ectopic bleeding starting at embryonic day 12.5 (E12.5) with the appearance of blood-filled lymphatics.33 The expression of 1 normal allele of Mcl-1 resulted in the birth of live pups, but with a 30% drop in the expected Mendelian ratio. Those animals that survived to adulthood had populations of bone marrow megakaryocytes visibly undergoing degeneration with nuclear pyknosis and cytoplasmic dysmorphology. These studies demonstrated that Bcl-xL and Mcl-1 play significant roles in megakaryocyte survival during maturation, platelet shedding, and half-life.
Apoptotic megakaryocytes and platelets have been identified in the stem cell–differentiation culture systems.21,38-40 The effects of modulating and/or inhibiting this pathway to increase megakaryocyte numbers should be further explored to optimize maturation of undamaged megakaryocyte populations. One study showed that the overexpression of Bcl-xL in human megakaryocytes increased proliferative capacity and survival.41 We have demonstrated that amplification of Bcl-xL increased the number of human megakaryocytes generated in vitro.42 Culturing Bak−/− murine fetal liver progenitors to megakaryocytes showed that in addition to 40% to 60% increased numbers of PPF megakaryocytes, these cells continued to be present in the culture over a 10-day period instead of following the normal pattern of disappearance after 5 days.37 A common pharmacologic inhibitor of the intrinsic apoptotic pathway is cyclosporine that prevents mitochondrial membrane permeabilization and apoptosis in a variety of cell types. This drug inhibited calcium ionophore–mediated apoptosis of platelets43 and phosphatidylserine exposure on agonist-stimulated platelets.44 The apoptotic machinery is highly conserved between species, but human megakaryocytes may be more sensitive to subtle changes in apoptotic protein levels. This is suggested by an interesting study of a family with mild autosomal-dominant thrombocytopenia.45 The defect was identified as a cytochrome c variant that displayed normal redox potential, but enhanced proapoptotic activity. Premature platelet shedding was observed in a patient’s bone marrow with the detection of intramedullary megakaryocyte nuclei and platelets. If human megakaryocytes have an exquisite sensitivity to apoptosis, it would be interesting to determine whether murine megakaryocytes are more resistant and whether this would explain why higher numbers of murine PPF megakaryocytes can readily be generated in culture as compared with human PPF megakaryocytes.
Surface receptor shedding, platelet clearance, and TPO regulation.
The presence of critical surface receptors on platelets and megakaryocytes, such as GPIbα and GPVI, are indicators of the health of the cell. The GPIbα (CD42b) subunit of the von Willebrand factor GPIb-V-IX receptor complex functions in cellular adhesion46,47 and GPVI is one of the collagen receptors involved in signaling.48 The cleavage of the N-terminal domain of GPIbα (termed glycocalicin49 ), which occurs during platelet storage, was identified on mouse platelets that were induced to undergo mitochondrial damage.50 This study showed that GPIbα cleavage could be inhibited by the broad-spectrum metalloproteinase (MMP) inhibitor GM6001, improving platelet clearance and function. A subsequent study showed that an MMP-induced mechanism of GPVI shedding also occurs in response to mitochondrial damage.51 Two membrane-bound MMPs, ADAM10 and ADAM17 or tumor necrosis factor-α–converting enzyme, were identified as responsible for the ectodomain shedding of these receptors.52,53 The shedding mechanism for GPIbα has recently been shown to also involve desialylation, priming the protein as a better MMP substrate.54 This study showed that even in the absence of receptor cleavage, desialylation caused platelets to be rapidly cleared from the circulation. Expression of the platelet sialidase Neu1 has been identified on the platelet surface following refrigeration, suggesting that treatment with sialidase inhibitors may improve survival of transfused platelets. This seminal work identified a long elusive mechanism for the in vivo steady-state regulation of TPO.55 This group showed that removal of desialylated platelets by the hepatic Ashwell-Morell receptor stimulated TPO messenger RNA expression, both in vivo and in vitro via Janus kinase 2 and signal transducer and activator of transcription 3 signaling, regulating bone marrow megakaryocyte production. Whether prosurvival and proapoptotic proteins have any role in determining surface sialic acid content is unknown. MMP inhibitors have been used in stem cell–differentiation systems showing slight increases in the numbers of CD42b+ megakaryocytes.20,38 The role of sialic acid loss and the effect of sialidase inhibitors on megakaryocyte maturation in the stem cell–differentiation systems will be of interest.
Generating megakaryocyte progenitor cells
In addition to in vitro injuries that can inhibit megakaryocyte maturation, a major problem with any cell culture system is the asynchronous nature of the differentiation process. For in vitro platelet production to be feasible for clinical usage, large numbers of PPF megakaryocytes will be required. One solution is the development of a stable progenitor intermediate or a self-renewing megakaryocyte progenitor pool that can be quickly driven to maturation for platelet production. Several approaches have been developed. A lentiviral approach was used to ectopically express the transcription factors MYC, BMI1, and the prosurvival protein Bcl-xL or BCL2L1 under the control of a doxycycline (dox)-inducible promoter in human ESC-derived megakaryocyte progenitors.41 With dox, the immortalized megakaryocyte progenitor cell line proliferated for up to 20 weeks and without dox, these progenitors completed terminal maturation and released platelets. Concerns of viral insertion-mediated mutagenesis raised safety issues, but established the feasibility of using self-renewing megakaryocyte progenitors for platelet production.
Another system manipulated the expression of the transcription factor GATA1, a master transcriptional regulator of megakaryopoiesis and erythropoiesis. This system was based on studies of patients with germline GATA1 loss-of-function mutations who had expanded populations of immature megakaryocytes in their bone marrow. GATA1-null mouse ESCs were differentiated to bipotential megakaryocyte erythroid progenitors (MEPs) that proliferated for several months without differentiating. These self-renewing progenitors were Gata1-deficient MEP (designated G1ME).56 When GATA1 expression was restored by retroviral expression of GATA1, G1ME cells differentiated into immature erythroblasts and megakaryocytes, but not further possibly due to nonphysiological overexpression of GATA1. An improved second-generation system was created, and these cells are designated G1ME2.57 This system functions under more physiologic conditions using a dox-inducible promoter for knocking down expression of GATA1 in mouse ESC-derived MEPs. In the presence of dox and GATA1 repression, G1ME2 cells expanded logarithmically for >40 days. Following dox removal, physiological levels of GATA1 were expressed and the G1ME2 cells differentiated into PPF megakaryocytes that released platelets. Of interest will be development of an analogous system derived from human PSCs.
Another system used a similar approach of manipulating endogenous genes by targeting the TPO receptor (c-mpl) with a reporter containing LoxP sites and expressing Cre recombinase driven by the PF4 promoter.58 In this system, mpl expression was detectable on bone marrow stem cells to the MEP stage of megakaryocyte maturation after which mpl was knocked out, resulting in undetectable expression in the late stages of megakaryocyte maturation and platelet generation. A twofold to fourfold increase in Lin−Sca+c-Kit+ (LSK) stem cells and all lineage progenitors except erythroid were present in the bone marrow and a 10-fold increase in platelets was detectable in the blood. A sixfold increase in the number of megakaryocytes was determined visually in bone marrow samples and ploidy was skewed to 32N. These data correlate with the observations that myeloproliferative disease patients with thrombocytosis have low levels of mpl expressed on their megakaryocytes and platelets.59,60 These data suggest that the final maturation of megakaryocytes may involve TPO-independent mechanisms such as gp130/interleukin-6 (IL-6)-dependent, Notch, and stromal cell–derived factor 1 (SDF1)/fibroblast growth factor 4–dependent signaling pathways.61 Of interest, the inflammatory gp130/IL-6 signaling pathway has been shown to coordinately regulate the expression of Bcl-xL and Mcl-1 in human myeloma cells.62 In addition, another inflammatory cytokine, IL-1α, has been implicated in a new mechanism of thrombogenesis involving megakaryocyte rupture and platelet release.63 At present, published strategies for in vitro growth of megakaryocytes rely on constant, high TPO expression throughout terminal differentiation. Whether targeting late events in developing megakaryocytes especially centered on the mpl/TPO axis would enhance megakaryopoiesis and thrombopoiesis in culture is untested.
Platelet manufacturing systems: providing cues for PPF and platelet release
To appreciate the process of translating the knowledge gained from stem cell–differentiation systems to the manufacture of platelets for transfusion, the reader is referred to an excellent, comprehensive review.64 This review discusses first-generation platelet manufacturing systems that set the groundwork for next-generation systems in recapitulating bone marrow environmental cues such as extracellular matrix composition, stiffness, shear stress, and endothelial cell contacts.65,66 Important recent advances in platelet manufacturing systems include visualization of PPF and platelet release by high-resolution live-cell microscopy in 3-dimensional (3D) microenvironments enabling resolution of PPF and platelet release in response to microenvironmental cues, flow conditions, and chemical and/or growth modulators. Advancements include quicker response times for PPF (6 hours to 2 hours), increased yield of PPF megakaryocytes (up to 90% in 1 system), and a programmable 3D silk bone marrow niche.65,66 Unfortunately, platelet yields remain at 10 to 30 per megakaryocyte. Stringent comparisons of bioreactor-generated platelets to adult, donor-derived platelets need to be performed using in vitro and in vivo systems. Shear force and exposure to appropriate biomaterials coating the various channels need to be perfected to generate platelets of physiological size distribution, surface marker expression, and in vivo half-life and functionality compared with donor-derived platelets.
Generating non-donor-derived platelets: a work in progress
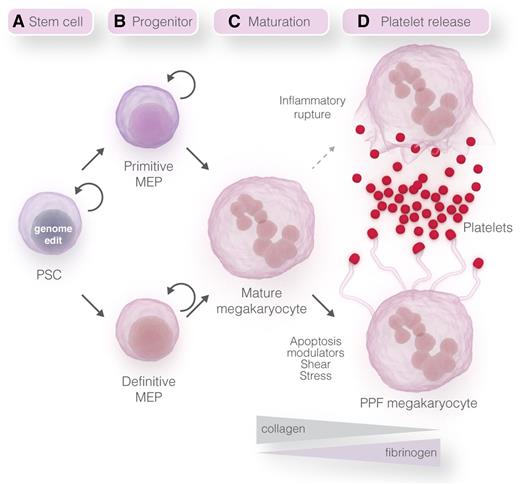
A hypothetical model with each of the components of a stem cell–differentiation system leading to platelet generation is depicted in Figure 1. A summary of each component, including challenges to overcome and possible solutions for improvements, will be discussed.
Stepwise model for platelet manufacturing. (A) Self-replicating human PSCs can be genetically manipulated using genome-editing technologies.67 (B) Current PSC-differentiation systems generate primitive MEPs and megakaryocytes,20,21 but recent protocol advancements79 may enable future systems to generate definitive-like MEPs and megakaryocytes. Self-replicating MEPs have been generated using PSCs with inducible systems to regulate the expression level of transgenes or endogenous transcription factors.41,57 (C-D) Megakaryocyte maturation and PPF may be induced using different matrices, modulators of apoptosis, and shear stress.65,66 Platelet release could also be induced from mature megakaryocytes by inflammatory induced rupture of the cell.63 Professional illustration by Somersault18:24.
Stepwise model for platelet manufacturing. (A) Self-replicating human PSCs can be genetically manipulated using genome-editing technologies.67 (B) Current PSC-differentiation systems generate primitive MEPs and megakaryocytes,20,21 but recent protocol advancements79 may enable future systems to generate definitive-like MEPs and megakaryocytes. Self-replicating MEPs have been generated using PSCs with inducible systems to regulate the expression level of transgenes or endogenous transcription factors.41,57 (C-D) Megakaryocyte maturation and PPF may be induced using different matrices, modulators of apoptosis, and shear stress.65,66 Platelet release could also be induced from mature megakaryocytes by inflammatory induced rupture of the cell.63 Professional illustration by Somersault18:24.
Stem cell choice
Pluripotent stem cells.
Human PSCs remain the most attractive stem cell source for generating non-donor-derived megakaryocytes and platelets because they are a renewable, inexhaustible source of cells that are amenable to genetic manipulation using genome-editing technologies.67,68 As discussed, these cells are an ideal source for generating self-replicating megakaryocyte progenitor cells to increase final megakaryocyte and platelet yield (Figure 1A). The creation of induced PSC-derived HLA-ABC−/− cells by transcription activator–like effector nuclease (TALEN)-mediated disruption of β2-microglobulin is already a reality for attempting to generate universal platelets.20 To increase megakaryocyte and platelet survival, genome editing of the apoptotic pathway may be a possibility as shown in the mouse system.36 Inducible and megakaryocyte transgene expression can also be performed using the PPP1R12C (AAVS1) “safe harbor” locus, which prevents deleterious off-target cloning and gene silencing that can occur during differentiation.69-72 Modulation of transcriptional master regulators, such as PU.1, can be used to eliminate unwanted myeloid cell fates71 that may contribute to the enzymatic injuries of megakaryocytes.
Primitive vs definitive megakaryocytes.
During different stages of normal development, distinct waves of hematopoiesis, designated primitive and definitive, occur within specific locations of the embryo (Figure 1B). Primitive progenitors develop from an extraembryonic mesodermal population that is specified to a hematopoietic fate in the yolk sac (reviewed in Baron and Fraser,73 Doulatov et al,74 Frame et al,75 and Tavian and Péault76 ). The yolk-sac or embryonic progenitors generate nucleated red blood cells (RBCs) expressing embryonic forms of globin, macrophages, and primitive megakaryocytes. The progenitor cells and megakaryocytes generated in the current PSC-differentiation systems are primitive, recapitulating this stage of normal embryonic hematopoiesis.68 The second wave of hematopoiesis, termed definitive, is initiated at specific sites in the arterial vasculature of a developing embryo, the best characterized being the ventral wall of the dorsal aorta in the caudal region that contains the aorta, gonad, and mesonephros.77 This program generates all of the hematopoietic lineages including long-term reconstituting hematopoietic stem cells, enucleated RBCs, myeloid cells, lymphocytes, and definitive megakaryocytes.77 Following specification in the aorta, gonad, and mesonephros, the hematopoietic stem cells colonize the fetal liver and subsequently transition to the bone marrow.
Recently, in vitro differentiation protocols have identified definitive-like hematopoietic programs that include hallmarks of lymphocyte potential, globin switching in RBCs, and multilineage hematopoietic engraftment in rodent reconstitution models.78,79 An extensive transcriptome profiling of megakaryocytes derived from each of these developmental stages has identified differentially expressed sets of genes that can be grouped into primitive and definitive gene profiles.27 Comparing embryonic, fetal liver, cord blood, and adult-derived megakaryocytes, this study highlights phenotypic and genotypic differences at these developmental stages and shows that ESC-derived megakaryocytes represent the primitive lineage. We have found that megakaryocytes generated from the newly described progenitor pool have a gene expression signature more similar to definitive than primitive megakaryocytes (X.S., M.P., P.G., D.L.F., personal observations). As megakaryocytes continue to be generated from newly discovered stem cell–derived progenitor pools, the most important end point will be functional analyses of the final platelet product. Platelets derived from the primitive lineage are larger and more reticulated than definitive cells80,81 and platelets in the embryo have important roles in nonhemostasis-related biologies such as the development of the lymphatic blood barrier.82 Platelets derived from cord blood and the neonate display hyporesponsiveness to various agonists, possibly due to deficiencies in specific signaling pathways.83-85 Crucial issues to be addressed will be the developmental stage equivalent of in vitro–generated megakaryocytes and platelets, and the impact of developmental differences on the therapeutic potential of the final product.
Self-replicating megakaryocyte progenitor cell
To generate the yield of platelets required for transfusion, self-replicating megakaryocyte progenitors will be essential for any platelet-generating system (Figure 1B). The human immortalized megakaryocyte progenitor cell line41 and murine G1ME2 cell line57 are proofs-of-principle for self-replicating progenitors that can be used to increase megakaryocyte yield. The advantages of these prototypic cell systems include (1) overcoming the asynchronous nature of cell differentiation due to a block at an early progenitor stage, (2) utilization of an inducible system for gene expression and differentiation, (3) ability to cryopreserve, enabling large numbers to be accessible for immediate use, and (4) generation from PSCs that have been genome edited for knockouts or knockdowns to enhance survival and eliminate nonmegakaryocyte cell fates.
Megakaryocyte maturation, PPF, and platelet release
In our very simplified schematic, one envisions these processes in a bioreactor-like system. One module may be designed for megakaryocyte maturation without PPF and another module designed for PPF and platelet release (Figure 1C-D). These criteria may be fulfilled by using different matrices in which a known inhibitor of PPF megakaryocytes is type I collagen whereas inducers of PPF megakaryocytes include fibrinogen, fibronectin, type IV collagen, and laminin. Examples of this inhibition and induction of PPF megakaryocytes were shown in the 3D silk platelet manufacturing system demonstrating their feasibility of use.65 In addition to matrices, designing the appropriate vasculature may become a reality using PSC-derived endothelial cells in which the identification of distinct endothelial lineages have already been described.86,87 Soluble triggers of PPF and platelet release have not been identified but putative mediators include SDF-1 and sphingosine-1-phosphate. The SDF-1/CXC chemokine receptor type 4 signaling axis has been shown to be involved in the migration and location of megakaryocytes to appropriate bone marrow niche locations for platelet release.88-90 A gradient of sphingosine-1-phosphate in the bone marrow vasculature has been shown to guide proplatelet processes for unidirectional shedding of platelets in the circulation.91,92 Platelet generation may also be possible without PPF megakaryocytes and triggered from mature megakaryocytes utilizing the newly described IL-1α rupture method.63
Conclusion
Despite major advancements in generating megakaryocytes from a variety of different stem cell sources and the development of platelet bioreactor systems, platelet yield and quality remain suboptimal for clinical use. A major stumbling block in this process is maintaining megakaryocyte viability during endomitosis and cytoplasmic maturation. Manipulation of the apoptotic pathway in PSCs may overcome some of these viability issues and improve megakaryocyte yield. In addition, manipulation of this pathway and elimination of nonmegakaryocyte lineages may help to overcome some of the enzymatic-driven injuries incurred to the megakaryocyte during cell culture. Megakaryocyte yield can be increased by the development of a self-replicating megakaryocyte progenitor system. Platelet yield may be enhanced by developing a system using progenitors derived from the definitive hematopoietic program. In all systems, assessment of platelet quality is essential and requires comparison with donor-derived platelets, which remain the gold standard. The minimal criteria for assessment of in vitro–derived platelets may include: (1) responsiveness to agonists using standard aggregometry, (2) function in thrombus formation in vivo, and (3) half-life determination. By utilizing the technological advancements in the fields of stem cell biology, genome editing, and bioengineering, the generation of in vitro–derived platelets will continue to move forward to becoming a clinical reality.
Authorship
Contribution: X.S. wrote and revised the manuscript, reviewed literature, and designed the figure; M.P. provided expertise in the field as well as scientific direction and revised drafts; P.G. provided scientific direction, revised drafts, and provided expertise in the field; and D.L.F. wrote and revised the manuscript and provided scientific direction and expertise in the field.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Deborah L. French, The Children’s Hospital of Philadelphia, 3501 Civic Center Blvd, CTRB 5014, Philadelphia, PA 19104; e-mail: frenchd@email.chop.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal