Abstract
Hematopoietic stem cells (HSCs) can be safely collected from the body, genetically modified, and re-infused into a patient with the goal to express the transgene product for an individual’s lifetime. Hematologic defects that can be corrected with an allogeneic bone marrow transplant can theoretically also be treated with gene replacement therapy. Because some genetic disorders affect distinct cell lineages, researchers are utilizing HSC gene transfer techniques using lineage-specific endogenous gene promoters to confine transgene expression to individual cell types (eg, ITGA2B for inherited platelet defects). HSCs appear to be an ideal target for platelet gene therapy because they can differentiate into megakaryocytes which are capable of forming several thousand anucleate platelets that circulate within blood vessels to establish hemostasis by repairing vascular injury. Platelets play an essential role in other biological processes (immune response, angiogenesis) as well as diseased states (atherosclerosis, cancer, thrombosis). Thus, recent advances in genetic manipulation of megakaryocytes could lead to new and improved therapies for treating a variety of disorders. In summary, genetic manipulation of megakaryocytes has progressed to the point where clinically relevant strategies are being developed for human trials for genetic disorders affecting platelets. Nevertheless, challenges still need to be overcome to perfect this field; therefore, strategies to increase the safety and benefit of megakaryocyte gene therapy will be discussed.
Overview: normal megakaryocytopoiesis, platelet production, and function
Hematopoietic stem cells (HSCs) are not only self-replicating but they are also pluripotent, thus capable of differentiating into 2 major lineages known either as lymphoid or myeloid blood cells.1 The myeloid lineage further differentiates into 3 distinct cell types known as myelocytes, erythrocytes, and megakaryocytes. Figure 1 is a schematic diagram that depicts the HSCs that become the progenitor cell committed to megakaryocyte differentiation, which remains capable of mitotic cell division2 although the proliferating diploid megakaryocyte progenitor cell gradually loses its capacity to divide and undergo endomitosis. Unique among the other cell types, a maturing megakaryocyte retains its ability to replicate DNA but neither the nucleus, cytoplasm, nor cell membrane divides resulting in a unique multiploidy (8N-128N) nucleated cell with a very complex internal membrane system, granules, and organelles.3 A mature megakaryocyte enters an apoptotic stage where the nucleus degrades and the cytoplasm and cell membrane fragments into ≤5000 proplatelets per cell.4 This ultimately leads to formation of circulating blood platelets that are small (3-µm diameter), anucleate, and discoid-shaped entities that play a fundamental role in hemostasis.5
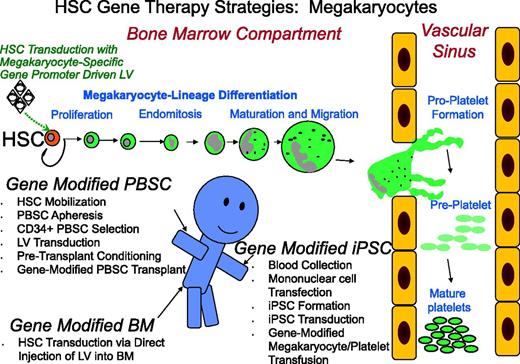
Promising strategies for megakaryocyte gene transfer. Displayed is a schematic diagram that summarizes 3 strategies currently being examined for megakaryocyte modification including: transplantation of cytokine-mobilized CD34+ PBSCs transduced with a lentiviral vector; direct injection of lentiviral vector into the bone marrow space to transduce HSCs; and lentiviral vector transduction of iPSCs dedifferentiated from peripheral blood mononuclear cells followed by transfusion of genetically altered platelets into the patient. Although each method focuses on modification of the HSCs with a lentiviral vector under the transcriptional control of a megakaryocyte-specific gene promoter, there is significant contrast in procuring the HSC target cell as well as different strategies for accomplishing megakaryocyte manipulation. HSC differentiation along the megakaryocyte lineage is depicted by stage from HSCs within the bone marrow to formation of proplatelets, preplatelets, and finally mature platelets within the vascular space. The cartoon person illustrates the routes of collection and transfusion of genetically modified cells and a potential point of injection for LV within the bone marrow space. PBSCs are all HSCs. BM, bone marrow; LV, lentivector.
Promising strategies for megakaryocyte gene transfer. Displayed is a schematic diagram that summarizes 3 strategies currently being examined for megakaryocyte modification including: transplantation of cytokine-mobilized CD34+ PBSCs transduced with a lentiviral vector; direct injection of lentiviral vector into the bone marrow space to transduce HSCs; and lentiviral vector transduction of iPSCs dedifferentiated from peripheral blood mononuclear cells followed by transfusion of genetically altered platelets into the patient. Although each method focuses on modification of the HSCs with a lentiviral vector under the transcriptional control of a megakaryocyte-specific gene promoter, there is significant contrast in procuring the HSC target cell as well as different strategies for accomplishing megakaryocyte manipulation. HSC differentiation along the megakaryocyte lineage is depicted by stage from HSCs within the bone marrow to formation of proplatelets, preplatelets, and finally mature platelets within the vascular space. The cartoon person illustrates the routes of collection and transfusion of genetically modified cells and a potential point of injection for LV within the bone marrow space. PBSCs are all HSCs. BM, bone marrow; LV, lentivector.
Evolution of megakaryocyte manipulation
A significant advance in furthering our understanding of platelets was realized with the use of molecular biological techniques for transfer of recombinant DNA for expression of platelet proteins within oncogenic promegakaryocyte transformed cell lines and nonmegakaryocytic cell lines transfected with recombinant DNA.6 It has been ≈30 years since researchers attempted to alter tissue-cultured megakaryocytes derived from transfection of HSCs.7 This work occurred simultaneously with reports of groundbreaking preclinical studies demonstrating gene transfer techniques useful for correction of inherited disorders.8-11 Results of the first clinical gene transfer trials for an immunologic disorder (adenosine deaminase–severe combined immunodeficiency) began ≈20 years ago,12 whereas it has only been ≈15 years since reports of successful genetic manipulation of human megakaryocytes were first published and clinical trials targeting the megakaryocyte lineage are still in the planning stages.13-15 This discrepancy of time is attributed to a scarcity of megakaryocytes in vivo which made it difficult to propagate and examine these cells in vitro. The ability to manipulate megakaryocytes was improved greatly when researchers developed methods to propagate and differentiate a sufficient number of megakaryocytes and platelets in vitro and in vivo as a result of the discovery of the c-mpl ligand (aka, thrombopoietin, megakaryocyte growth, and development factor).16,17 Initial preclinical research focusing on megakaryocyte manipulation used early-generation recombinant viral vectors and nonviral gene transfer techniques that proved moderately effective at genetically modifying tissue-cultured HSCs and megakaryocytes derivatives. Today, technological advances that proved successful for recent human gene therapy of other hematologic disorders18,19 are being adopted to help design clinical protocols for the first human trials aimed at modifying human megakaryocytes to correct inherited bleeding disorders (eg, Glanzmann thrombasthenia [GT], Bernard-Soulier syndrome [BSS], and hemophilia, which will be discussed in greater detail in this review).
Gene therapy targeting the megakaryocyte lineage
Genetic therapies aimed at modifying megakaryocytes can potentially be used to improve a wide variety of disorders because platelets play a role in several physiological events (eg, hemostasis, immune response, and wound healing) as well as pathological conditions (eg, atherosclerosis, cancer, sepsis, and thrombosis).20 To participate in these biological processes, platelets use a plethora of biologically active molecules on the surface, in the cytoplasm, and within granular compartments that mediate platelet function.6 Platelets normally circulate quiescently (nonreactive with other blood cells or the vasculature) until they become activated upon stimulation by physiological agonists of platelet activation (eg, adenosine 5′-diphosphate, epinephrine, thrombin), adhere to subendothelial matrix molecules (collagen, von Willebrand factor) at the site of a vascular injury, change shape, and release the contents of their granules. Because platelets are anucleate, a strategy aimed at replacing or editing defective genes involved in normal platelet function or inducing ectopic expression of genes to manipulate platelet function would have to be focused on altering the genetics of HSCs, progenitor cells, or megakaryocytes depending upon the optimal length of time the gene product should be expressed. For example, a strategy aimed at using platelets to deliver an antioncogenic agent to treat cancerous tumors may try to provide a chemotherapy treatment of a limited time. In contrast, a protocol with the goal of long-term correction of an inherited platelet defect may likely strive for permanent genetic manipulation of the HSC because human megakaryocytes have a lifespan of ≈5 days when undergoing polyploidization, maturation, and platelet formation.21
There are several megakaryocyte-specific gene promoters that could potentially direct transgene transcription including: members of the glycoprotein (GP) GPIBA-GPIX-GPV complex,22 ITGA2B (aka, integrin αIIb, GPIIb),23 GPVI,24 c-mpl,25 and platelet factor 4 (PF4).26 These promoters bind GATA-1, Ets (Fli-1), and FOG-1 factors that induce transcription in early and mid stages of megakaryocytopoiesis.27,28 For example, PF4 is expressed at high levels during megakaryocytopoiesis and stored within platelet α-granules.29 Its gene promoter26 and distal regulatory regions30 are well characterized and have been shown to be useful for controlling megakaryocyte-specific transgene expression. To date, the most common megakaryocyte-specific gene promoters used within gene transfer vectors used for megakaryocyte modification include: GP1BA, ITGA2B, and PF4 because they have been shown to consistently drive moderate- to high-level protein expression preferentially within megakaryocytes (see Table 1).31-33 These gene promoters could potentially be used to drive megakaryocyte-specific transgene expression within the confines of a variety of gene transfer vectors that have been characterized for their ability to efficiently and safely express the transgene product including: recombinant lentiviral, adenoviral, and adeno-associated viral vectors as well as plasmid DNA.34
Advantages and disadvantages of new strategies to correct hemophilia A
| Gene vector . | Target . | Gene promoter . | FVIII . | Reference . | Advantages . | Disadvantages . |
|---|---|---|---|---|---|---|
| AAV | Endothelium liver | Liver specific | BDD FVIII modified | 61 | +No pre-tx conditioning | −Vector size limitation |
| +Shown safe in humans | −Use only in patients without AAV inhibitor and without FVIII inhibitor | |||||
| +No reports of mutagenesis | −Cell death ends treatment | |||||
| LV | HSC CD34+ PBSCs | Nonspecific CMV | BDD FVIII modified | 63 | +Use with AAV inhibitors | −Submyeloablative conditioning required |
| −Mutagenesis risk | ||||||
| +Theoretically 1 treatment | −Use only without FVIII inhibitor | |||||
| LV | HSC CD34+ PBSCs | Meg-GPIBA | BDD FVIII modified | 67 | +Use with AAV and FVIII inhibitors | −Submyeloablative conditioning required |
| +Theoretically 1 treatment | −Mutagenesis risk | |||||
| −GPIBA gene promoter associated with low plt production | ||||||
| LV | HSC BM | Meg-GPIBA | BDD FVIII | 72 | +No pre-tx conditioning | −Target cell not purified for transduction |
| −Mutagenesis risk | ||||||
| +Use with AAV and FVIII inhibitors | −GPIBA gene promoter associated with low plt production | |||||
| +Theoretically 1 treatment | −Feasibility in humans? | |||||
| LV | HSC CD34+ PBSCs | Meg-ITGA2B | BDD FVIII | 31 | +Use with AAV and FVIII inhibitors | −Submyeloablative conditioning required |
| +Normal plt production | −Mutagenesis risk | |||||
| Gene vector . | Target . | Gene promoter . | FVIII . | Reference . | Advantages . | Disadvantages . |
|---|---|---|---|---|---|---|
| AAV | Endothelium liver | Liver specific | BDD FVIII modified | 61 | +No pre-tx conditioning | −Vector size limitation |
| +Shown safe in humans | −Use only in patients without AAV inhibitor and without FVIII inhibitor | |||||
| +No reports of mutagenesis | −Cell death ends treatment | |||||
| LV | HSC CD34+ PBSCs | Nonspecific CMV | BDD FVIII modified | 63 | +Use with AAV inhibitors | −Submyeloablative conditioning required |
| −Mutagenesis risk | ||||||
| +Theoretically 1 treatment | −Use only without FVIII inhibitor | |||||
| LV | HSC CD34+ PBSCs | Meg-GPIBA | BDD FVIII modified | 67 | +Use with AAV and FVIII inhibitors | −Submyeloablative conditioning required |
| +Theoretically 1 treatment | −Mutagenesis risk | |||||
| −GPIBA gene promoter associated with low plt production | ||||||
| LV | HSC BM | Meg-GPIBA | BDD FVIII | 72 | +No pre-tx conditioning | −Target cell not purified for transduction |
| −Mutagenesis risk | ||||||
| +Use with AAV and FVIII inhibitors | −GPIBA gene promoter associated with low plt production | |||||
| +Theoretically 1 treatment | −Feasibility in humans? | |||||
| LV | HSC CD34+ PBSCs | Meg-ITGA2B | BDD FVIII | 31 | +Use with AAV and FVIII inhibitors | −Submyeloablative conditioning required |
| +Normal plt production | −Mutagenesis risk | |||||
BM, bone marrow; CMV, promoter of the cytomegalovirus; LV, lentiviral vector; Meg, megakaryocyte-specific; plt, platelet; tx, transplant.
Each gene transfer technique has unique advantages and disadvantages depending partially upon vector used and the disorder that is being treated (summarized in Table 1). This report will focus mainly on use of a recombinant lentiviral gene transfer vector because it has been shown to deliver remarkable results in human clinical trials that target HSCs. This vector can accommodate relatively large complementary DNA (cDNA) cassettes (≈10 kb), integrate stably into the genome within nondividing HSCs, and appears relatively safe and efficient for sustained gene transfer for a variety of inherited genetic disorders.18,19
Correction of inherited platelet defects
Defects of distinct platelet proteins are very rare (≈1:1 000 000 individuals); however, taken collectively, a molecular genetic defect in a gene that plays a role in normal platelet function occurs in ≈1:10 000 individuals usually manifesting itself in the form of uncontrolled bleeding.35 A recent review by Nurden and Nurden elegantly illustrates several known genetic defects affecting platelet proteins that may be ideal candidates for gene therapy including surface molecules (TMEM16F, Scott syndrome), cytoplasmic (STIM1/ORAI, Stormorken syndrome) and structural proteins (WASP, WIPF1, Wiskott-Aldrich syndrome) as well as granule constituents (NBEAL2,GFI1B, gray platelet syndrome).36 Disorders resulting from platelet defects are commonly referred to as “benign” because uncontrolled bleeding frequently responds favorably to treatment with platelet transfusions, use of antifibrinolytic agents, and recombinant factor VIIa (FVIIa).37 However, patients can become refractory to platelet transfusions and infusion of these medications can be costly and short-lived.38 Allogenic bone marrow transplant has been used successfully to correct dogs and humans with GT,39,40 although transplant-related complications (eg, graft-versus-host disease, graft failure) has limited the use of this treatment.41,42
Gene transfer into cytokine-mobilized CD34+ peripheral blood stem cells (G-PBSCs) from 2 GT patients served as the first model to test the feasibility of correcting a platelet defect with HSC gene transfer using a retroviral vector under the transcriptional control of a megakaryocyte-specific ITGA2B gene promoter driving expression of a normal human ITGB3 replacement cDNA gene cassette.43 Results from that study showed that retroviral vector transduction of CD34+ G-PBSCs, leading to expression of 34% of normal levels of a functional integrin αIIbβ3 receptor on the surface of 19% progeny megakaryocytes, was sufficient to permit cells to mediate retraction of a fibrin clot in vitro.43 The ability of HSC gene replacement therapy to correct a platelet defect in vivo was observed when hemostasis was improved in mice affected with GT following expression of ≈10% of normal integrin receptor levels of a functional hybrid murine αIIb-human β3 complex on the surface of ≈50% of megakaryocytes derived from a transplant of Itgb3(−/−) bone marrow transduced with the ITGA2B gene promoter-controlled retroviral vector encoding human β3 into mice preconditioned with irradiation causing complete myeloablation.44 Because GT is considered a benign disorder, a mild (clinically relevant) strategy using submyeloablative pretransplant conditioning was developed. It was observed that hemostasis could be established for at least 5 years in a canine (large animal) model of GT following autologous transplant of CD34+ G-PBSCs transduced with an ITGA2B gene promoter-controlled lentiviral vector driving expression of human αIIb coupled with a drug-selection gene methyl-guanine methyl-transferase (MGMT) under the transcriptional control of a tissue-nonspecific gene promoter.45 Thus, HSCs expressing MGMT were enriched in vivo by treatment of animals with Carmustine (O6-benzyl guanine), which ultimately resulted in de novo expression of 6% of normal levels of a functional human αIIb-canine β3 hybrid receptor on the surface of 10% circulating blood platelets.45 These modest levels of gene transfer allowed platelets to adhere to the receptor’s major ligand (fibrinogen), form measurable aggregates, and mediate retraction of a fibrin clot in vitro. Remarkably, improved hemostatic function was evident with observations of ≈135-fold reduced blood loss and improved buccal bleeding time decreased to 4 minutes compared with >20 minutes (experimental end point for untreated GT dogs) for at least 5 years after HSC transplant.
A second gene replacement strategy for GT has been recently reported that used gene transduction of induced pluripotent stem cells (iPSCs) with a vector expressing human β3 under the transcriptional control of the megakaryocyte-specific Gp1BA gene promoter. The iPSCs were dedifferentiated and immortalized from peripheral blood derived from 2 GT patients.32 Similar to HSC gene transfer and transplant, this approach resulted in the de novo expression of 50% of normal levels of a functional αIIbβ3 receptor on the surface of megakaryocytes in vitro.32 The results from this work indicate that transfusion of gene-modified autologous platelets derived from iPSCs could provide additional elements of safety for a gene transfer protocol because the potential for the patient to develop mutagenesis and oncogenesis due to pretransplant conditioning reagents and random insertion of a gene transfer vector into the genome is greatly reduced by transfusion of anucleate platelets. In addition, transplant of autologous platelets with 1 new receptor may decrease immune-mediated recognition and destruction of transfused platelets as observed with transfusions from unrelated donors. However, treating uncontrolled bleeding events with this strategy would likely require a lifetime of multiple transfusions with a sufficient quantity of platelets derived from gene-modified iPSCs, whereas in contrast, HSC gene transfer and transplant should theoretically only require 1 transfusion.
The feasibility of HSC gene therapy aimed at modifying megakaryocytes was confirmed useful for another rare inherited platelet defect causing BSS. The investigators first used an ITGA2B gene promoter-controlled lentiviral vector encoding GP1bα in tissue-cultured human cells in vitro.46 This was followed by work that showed improved platelet structure and function following GP1BA lentiviral vector gene transduction of bone marrow that was transplanted into a murine model of BSS.47
Arterial occlusive disorders are a leading cause of human morbidity. Thus, platelets may also be useful for delivery of antithrombotic agents to sites of occlusion. There is 1 report of the ectopic expression of urokinase-type plasminogen activator in platelets of mice affected with the inherited platelet defect know as Quebec platelet disorder.48 This group showed that expression of fibrinolytic proteins in platelets could be used to favorably alter the hemostatic balance at sites of thrombosis via ectopic expression of murine urokinase-type plasminogen activator within transgenic mice where transgene expression was driven by the megakaryocyte-specific PF4 gene promoter. Results showed that targeted expression and storage of urokinase in the platelets of transgenic mice altered platelet biology and a bleeding diathesis similar to that seen in patients with Quebec platelet disorder. This confirmed the role of ectopic urokinase expression as the etiology of this inherited disease. Remarkably, the mice were resistant to the development of occlusive carotid artery thrombosis in the absence of systemic fibrinolysis and displayed rapid resolution of pulmonary emboli. Furthermore, transfusion of urokinase-expressing platelets into wild-type mice prevented formation of occlusive arterial thrombi. In summary, results of that study indicate feasibility for delivering fibrinolytic agents to sites of thrombus formation following targeted storage of urokinase in platelets indicating a potential viable strategy to prevent thrombosis and hemorrhage.
Ectopic delivery of coagulation factors from platelets
Hemophilia A is a severely debilitating and deadly hemorrhagic disorder (incidence ≈1:5000 males) linked to molecular genetic defects in the plasma protein, coagulation FVIII.49,50 Plasma-derived and recombinant factor concentrates are currently available from numerous sources for administration (frequently several times per week) to treat serious bleeding episodes. Infusion of ≥1% of normal FVIII levels has shown dramatic improvement in hemostasis. In addition, nonfactor products including desmopressin have also been used, depending on the clinical situation and severity of FVIII deficiency. New treatment strategies are being investigated that use permanent transfer of a replacement gene transfer into HSCs to provide continuous production of FVIII secreted precisely at the site of vascular injury from activated platelets (Table 1). This result could dramatically improve the quality of life for patients and may substantially reduce the cost of clinical care for spontaneous uncontrolled bleeding events.51
Initial human trials for FVIII gene transfer involving the implantation of transformed fibroblasts and infusion of recombinant viral vectors targeting the liver failed to produce sustained correction of the hemophilia A bleeding diathesis.52,53 However, recent success of gene therapy for hemophilia B treatment with adeno-associated viral vector (AAV)-mediated factor IX (FIX) expression within the liver for secretion into the blood indicates that it may be feasible to develop a similar strategy for treatment of hemophilia A.54 Gene transfer for hemophilia A appears to be a greater challenge than hemophilia B for a variety of reasons including (1) locating a gene transfer vector that can accommodate the large B-domain deleted (BDD) FVIII cDNA (4 kb)55 compared with the small FIX cDNA (1.5 kb), (2) achieving adequate levels of transgene expression, and (3) preventing/averting a frequent complication of the development of anti-FVIII immunity as well as immune recognition of the coat protein of the AAV gene transfer vector (which is also a potential problem for FIX gene transfer). Table 1 is a summary of some of the advantages and disadvantages for a few of the recent strategies that have been proposed that promote expression of human FVIII for correction of hemophilia A. There have been reports that inducing de novo FVIII expression within the liver endothelial cells by either IV infusion of naked DNA56 or a new generation of AAV equipped with less immunogenic coat proteins and a vector encoding small active forms of FVIII cDNA (that can meet the 4.4-kb packaging capacity of AAV) shows promise for endothelial synthesis and secretion of FVIII into the plasma to treat hemophilia A.57-61 Nonetheless, as with AAV clinical trials for hemophilia B, targeting the liver for FVIII expression will likely exclude hemophilia A patients with preexisting antibodies to the AAV viral capsid (≈40%), individuals who have already developed or could develop inhibitory antibodies to plasma FVIII (≈30%), and persons with preexisting liver disease or damage due to acquisition of hepatitis or HIV from contaminated blood products from factor replacement therapy.51 Thus, as a potentially viable alternative approach to advert the challenges imposed by AAV-mediated liver-targeted therapies for hemophilia A, we and others hypothesize that autologous transplant of HSCs transduced with a lentiviral vector (≈10-kb DNA packaging capacity) encoding replacement FVIII cDNA may be an ideal strategy for correction of hemophilia A within humans (Figure 1). One group proposes to transduce HSCs with a lentiviral vector under the transcriptional control of a tissue-nonspecific gene promoter that expresses FVIII within all hematopoietic cell lineages.62,63 Because activated blood platelets mediate the primary response to vascular injury by adhering to a wound site and secreting biologically active proteins,20 it is speculated that use of a megakaryocyte-specific gene promoter that confines synthesis and storage of FVIII within platelets may be a more tailored approach for providing continuous, locally inducible treatment of maintaining hemostasis precisely at the site of vascular injury for hemophilia A. Support for this approach has been observed in preclinical studies that showed HSC gene transfer targeting expression within human megakaryocytes resulted in the trafficking of a biologically active form of human FVIII into the α-granule compartment of human platelets derived from HSCs xenotransplanted in mice.64 Further investigations of HSC lentiviral-mediated gene transfer demonstrated that platelet FVIII is capable of establishing hemostasis in murine and canine models of hemophilia A (even in the presence of inhibitory antibodies in mice and without eliciting the formation of antibodies in a line of hemophilia A dogs known to readily form inhibitors to human FVIII).31,65-67 These results suggest that platelets engineered to store FVIII have great potential for establishing hemostasis in humans with hemophilia A. The data indicate that platelet FVIII may be essential for patients affected with inhibitory antibodies to FVIII, which would likely cause their exclusion from clinical trials for liver-targeted genetic therapy or HSC tissue nonspecific expression of FVIII.68 It is noteworthy that Du et al have shown that a lentiviral vector using the ITGA2B gene promoter driving expression of a molecule encoding a fragment of the VWF propeptide and D2 domain (which helps traffic the molecule for storage within α-granules) fused to BDD FVIII successfully confined a majority of FVIII specifically within the platelet α-granule compartment of platelets to establish hemostasis for canine hemophilia A.31,69,70 These results indicate that the addition of an α-granule targeting peptide may add an additional level of safety for treating patients with preexisting inhibitory antibodies because sequestration of FVIII within platelet α-granules may help to concentrate FVIII within granules and prevent leakage of FVIII into the plasma from the cytoplasmic compartment. This result is consistent with studies performed in mice affected with von Willebrand disease that stored FVIII less efficiently within α-granules due the absence of von Willebrand factor (carrier protein for FVIII), which helps traffic FVIII into the granule compartment and protects FVIII from degradation.71
Wang et al have preliminary data in mice showing that it may be feasible to directly inject bone marrow with lentivector encoding FVIII under the transcriptional control of the megakaryocyte-specific GPIBA gene promoter (Figure 1).72 One advantage of this approach is that the mice did not require pretransplant conditioning with submyeloablative reagents (Table 1). Albeit, because the marrow cavity consists of several cell types, it is likely difficult to control the precise cell type that the viral vector will transduce. It also remains to be seen whether large animals and hemophilia patients (who encounter serious bleeding from the joints) could tolerate direct injection of vector into their bones.
Secretion of antioncogenic agents from platelets
Platelets have been observed to play a significant role in cancer. They can adhere directly to solid tumors leading to evasion from recognition and destruction by immune cells. Platelets have also been observed to release growth factors and proangiogeneic agents that promote tumor growth and metastasis.73 Thus, there is growing interest in developing strategies that permit platelets to synthesize, store, and deliver antioncogenic agents to cancer cells in an effort to confine chemotherapy to the tumor site. One report has demonstrated positive results following transfusion of platelets that were preloaded with chemotherapeutics or nanoparticles that inhibit oncogenesis.74 Another group showed that CD34+ HSCs transduced with a lentiviral vector under the transcriptional control of a tissue nonspecific (CMV) gene promoter driving expression of cDNA encoding an antiangiogenic agent, tumstatin. The HSCs successfully differentiated into megakaryocytes that synthesized, stored, and released tumstatin from platelet α-granules that caused an antiangiogenic effect on lung (A543) tumor cells in vitro.75 A recent report showed that the use of platelet interleukin-24 derived from HSC gene transfer was associated with the inhibition of melanoma solid tumor growth in mice.76 Each study demonstrates the “proof-of-concept” that it is feasible to equip platelets with antioncogenic agents to inhibit tumor growth. Because cancer is an acquired disease, gene transfer strategies using platelets engineered to express antioncogenic agents could be improved greatly with the ability to discontinue the treatment. Specifically, transfusion of an individual with genetically engineered platelets (with a life span of 10 days) may be preferable to infusion of HSCs modified to express the antioncogenic agent because it appears logical that the treatment should only be given as long as the cancer persists. Thus, recent studies describing transfusion of platelets derived from iPSCs or HSCs modified with gene transfer vectors indicate the potential feasibility toward accomplishing this goal.32,77,78 Current analysis of the field indicates that it is essential to achieve further improvement for in vitro production of platelets (especially to increase the number and quality of platelets for transfusion) to make this strategy clinically feasible.79 The authors of that article surmised that it may be more advantageous to infuse megakaryocytes (rather than platelets) derived from iPSCs. The megakaryocytes were observed to congregate within murine lungs where the maturation into platelets occurred. However, transplantation of genetically altered megakaryocytes could potentially pose a risk for insertional mutagenesis and oncogenesis resulting from infusion of a nucleated cell that has been genetically altered by integration of a lentiviral vector randomly into the genome. Furthermore, mutagenesis appears less of a risk by transfusion of anucleate platelets rather than infusion of megakaryocytes derived from iPSC cells that have been created by dedifferentiation of mature peripheral blood mononuclear cells to iPSCs with oncogenes. Nevertheless, optimism is high that these technical issues will be resolved.
Current challenges for modifying megakaryocytes and precursor cells
There are several issues that researchers had or will have to contend with to translate platelet gene therapy from bench to bedside. Some of the challenges are common to protocols that use lentiviral vector-mediated gene transfer (eg, concern of potential side effects from pretransplant conditioning regimen, insertional mutagenesis, development of an acquired immune response to the transgene product). Other challenges reported appear to be specifically associated with targeted genetic modification of the megakaryocyte lineage (eg, resulting in altered platelet function and decreased platelet production related to ectopic transgene expression).80
Submyeloablative pretransplant conditioning regimen
Some of the first successful gene therapy trials for X-linked severe combined immunodeficiency syndrome (X-SCID) involved the transfer of a gene (γc) that imparted a survival advantage to HSCs for genetic disorders affecting a patient’s ability to mount an immune response.81 This permitted the trial participants to forego pretransplant conditioning with chemotherapeutic agents that destroy untransduced stem cells to create a niche in the bone marrow for transplanted cells, thus improving the transduction efficiency and long-term gene marking in vivo. It is noteworthy that most of the genes examined in preclinical trials for modification of megakaryocytes for inherited bleeding disorders do not appear to impart a survival advantage to HSCs.6,34 Thus, preclinical trials in large animal models for GT and hemophilia A have used submyeloablative pretransplant conditioning with reduced-intensity total body irradiation or chemotherapeutic agents (eg, busulfan) with limited toxicity to HSCs as has been used for allogeneic “mini” bone marrow transplant protocols to help improve the percentage of HSCs carrying the transgene that engraft, while simultaneously limiting the risk of death from complete marrow ablation.31,45 The large animals (dogs) that received an autologous transplant of lentiviral vector-transduced CD34+ G-PBSCs appeared healthy at least until the experimental end point of 3 to 5 years after transplant, suggesting that the use of the submyeloablative conditioning protocol is likely safe for use in humans. Concerns have been expressed against the use of chemotherapeutic pretransplant conditioning regimens for HSC gene therapy for inherited bleeding disorders considered to be “benign.”68 Thus, they are likely the first clinical trials that demonstrate success will be performed on adult hemophilia A patients who have no treatment options for severe bleeding episodes due to development inhibitory antibodies to FVIII. A positive outcome in these patients showing low incidence of side effects as a result of the protocol will likely help to diminish concerns for use of a submyeloablative conditioning regimen.
Insertional mutagenesis
The risk vs benefit ratio for genetic modification for megakaryocytes for “benign” inherited bleeding disorders requires evidence of greater benefit and/or reduced risk of harm from the HSC gene transfer and transplant protocol compared with treatment offered by previous gene transfer trials for patients with inherited disorders with no treatment options.82 Retrovirus-based vectors have been improved and used for platelet gene therapy protocols because these constructs mediated the first successful human gene therapy trials in individuals affected with a hematologic disorder, X-SCID.83 Because all retroviral vectors incorporate randomly into the host genome, use of these constructs places the patient at some risk for insertional mutagenesis. For example, 3 of 10 patients treated with gene therapy for X-SCID developed leukemia as a result of mutagenesis of the patient’s genome.84 For 2 of the patients, an oncoretroviral vector (based upon the Moloney murine leukemia virus) inserted into and activated a T-cell proto-oncogene (LMO-2).85 X-SCID may be particularly susceptible to leukemogenesis because correction of the γ (c) gene defect confers a survival advantage to the transduced cells; this, plus alterations in genes such as LMO-2 that control cell division, may result in uncontrolled growth of cells that have a survival advantage.85,86 Another limitation of oncoretrovirus vectors is that they only integrate into cycling cells, and a majority of normal HSCs cycle very slowly (30 days per cycle on average). Because the efficiency of HSCs transduced with this system is low, it is not surprising that the first successful gene therapy clinical trial was for a disorder in which the transgene product provided a growth advantage for transduced HSCs.87 Recombinant lentiviral vectors (eg, HIV-1) are unique retroviruses that have become more useful for gene therapy of hematologic disorders because they can transduce nondividing cells.18,19 Second, compared with early retroviral vectors, lentiviral vectors appear to have improved safety with more of a propensity to insert into nontranscribed regions of the genome.88 Third, the use of endogenous gene promoters rather than viral gene promoters and enhancers appears to have helped increase safety as supported by analysis of the latest HSC human gene therapy trials, which have yet to report any adverse side effects due to insertional mutagenesis leading to oncogenesis.18,19
Immune response to transgene product
The introduction of a normal replacement gene or protein always raises the concern for the development of an acquired immune response to the transgene product. One mouse (n = 21) and 1 dog (n = 3) transplanted with HSCs transduced with lentiviral vector encoding β3 and αIIb, respectively, developed an immune response to the integrin receptor that led to severe destruction of genetically altered platelets.44,45 This outcome demonstrates that expressing foreign proteins within platelets does not guarantee tolerance or privilege against immune recognition. Fortunately, a standard treatment of diminishing immune-mediated destruction of human platelet transfusions (transient infusion of IV γ globulin “IVIgG” and corticosteroids) effectively diminished clearance platelets in animals that developed an immune response to de novo synthesis of αIIbβ3 on the surface of platelets derived from lentiviral vector transduced HSCs.33,34 Although this indicates that IVIgG may subside an immune response if it occurs during human clinical trials to correct GT, it is likely that an ideal candidate for the first clinical trial would be an individual who has not generated an immune response following multiple transfusions of donor platelets or a GT patient classified as type II or variant (who expresses residual levels of a dysfunctional form of αIIbβ3) because their immune system has been previously exposed to the αIIbβ3 without consequence. It appears remarkable that 20 of 21 mice and 2 of 3 dogs did not generate an immune response to de novo αIIbβ3 expression, indicating that it may be sufficient to express the least alloantigenic form of αIIbβ3 rather than custom design integrin subunits to match the genotype of each patient. Other preclinical data indicate that immune-mediated recognition of genetically altered platelets is potentially a greater risk for correction of GT with αIIbβ3 (expressed on the surface) compared with correction of hemophilia A where platelet-derived FVIII (sequestered within the cytoplasm and α-granules) has not been observed to elicit a detectable immune response in mice and dogs.31,66 This result is further supported by work demonstrating that platelet FVIII has been shown to improve hemostasis in hemophilia A mice that were conditioned to develop high titer inhibitory antibodies to FVIII.65,89 In summary, previous studies indicate that physical location of the transgene product could be related to the relative risk of developing immune response leading to destruction of genetically altered platelets; however, the precise mechanism for these phenomena remains to be determined.
Transgene expression causing altered platelet function
There has been concern raised that platelet-derived FVIII can affect normal platelet function by causing the formation of unstable blood clots. One report examined clot response to laser injury in both cremaster arterioles and venules in mice either infused with FVIII or transgenic for platelet-derived FVIII.90 In both sets of vessels, platelet FVIII was equally as effective as infused plasma FVIII. Temporal and spatial differences were observed in fibrin and platelet accumulation within clots depending on how FVIII was delivered. These differences were attributed to the temporal and spatial distribution of the α-granular–released FVIII within a developing clot that resulted in an increased frequency and size of embolic events seen with platelet FVIII. That result may have negative implications for the use of platelet FVIII in gene therapy for hemophilia A. However, although there were no formal analyses performed to detect emboli, there were no reported observations of increased size or frequency of embolic events occurring in mice or dogs affected with hemophilia A following successful transplant with HSCs transduced with a lentiviral vector expressing FVIII under the transcriptional control of the ITGA2B gene promoter.31,66
Transgene expression causing decreased platelet production
There has been at least 1 report of decreased platelet production following HSC gene transfer targeting ectopic expression of proteins within the megakaryocyte lineage of mice.80 This observation could be potentially of great significance because use of a megakaryocyte-specific or lineage-nonspecific gene promoter that induces synthesis of high levels of foreign proteins within megakaryocytes may have a negative effect on platelet production with effects that are not fully realized. The researchers of that study concluded that it may be more beneficial to express low levels of a protein of high specific activity. Consistent with that hypothesis, each study using a lentiviral vector driven by the ITGA2B gene promoter for platelet-targeted (ITGA2B, ITGB3, FVIII, FIX) transgene expression within murine and canine models for GT and hemophilia demonstrated very modest to moderate levels of protein synthesis, resulting in the ability to establish hemostasis within these animals for a prolonged period whereas platelet production levels were not affected.31,43,45,66 This modest level of FVIII synthesis could be attributed to the use of a human gene promoter within animal models, although use of the same ITGA2B gene promoter-controlled lentivectors within primary human HSC cells in tissue culture also resulted in modest levels of transgene expression.13,43,64 Based upon previous results, it is likely that use of the ITGA2B gene promoter will continue to drive moderate transgene transcription within human megakaryocytes in vivo that should be sufficient to improve hemostasis, whereas megakaryocytopoiesis and platelet production should not be affected with use of this vector in humans.
Summary
Significant advances in gene therapy (resulting from improved methods for HSC collection, transduction, and transplantation), as well as the development of a better understanding of the process of megakaryocytopoiesis, platelet production, and lineage-specific gene transfer, have all impacted current research investigations that aim to manipulate megakaryocytes and HSC precursors. Currently, there are at least 3 plausible strategies that target HSC gene transfer aimed at specifically modifying the megakaryocyte lineage, including lentiviral transduction of HSCs in the form of CD34+ PBSCs, bone marrow, and iPSCs (Figure 1).31,32,45,72 This work has furthered our efforts to achieve the ultimate goal of correcting inherited genetic defects of platelets and permitting platelets to deliver therapeutic agents directly to the site of injury. There remains an endless challenge to increase the safety and benefit from HSC gene transfer that will likely concentrate on developing strategies that obtain high transduction and gene marking efficiencies with low frequency for insertional mutagenesis. There is also a goal to decrease the potential for deleterious side effects through the development of pretransplant conditioning regimens that use mild but effective reagents that obtain optimal engraftment of genetically modified HSCs. There are also aspirations to produce a greater number of high-quality platelets from iPSCs that should increase the feasibility of this strategy to be used for clinical trials. Genetic therapy aimed at the manipulation of the megakaryocyte lineage will continue to strive for adequate protein synthesis and storage within progeny platelets without affecting the delicate balance of normal platelet production and function. Notwithstanding the current issues of the field, it is undoubtedly an exciting time to be involved in research aimed at megakaryocyte and megakaryocyte precursor gene therapy because the first human clinical trials targeting platelets appear to be on the near horizon.
Acknowledgments
Some of the research studies described in this review were supported by the following grants: National Institutes of Health–National Heart, Lung, and Blood Institute (NIH-NHLBI) R01 HL-68138 (D.A.W.), NHLBI Gene Therapy Resource Program (Indiana University Lentiviral Vector Production Laboratory) RSA 1253 (D.A.W.), and Production Assistance for Cellular Therapies (Boston Children's Hospital Laboratory) NHLBI 00085/Wilcox; American Heart Association (Northland Affiliate) Beginning Grant-in-Aid 0160441Z and Grant-in-Aid 0755827Z (D.A.W.); and generous gifts from the Children’s Hospital Foundation (D.A.W.), Midwest Athletes Against Childhood Cancer Fund (D.A.W.), John B. & Judith A. Gardetto (D.A.W.), Glanzmann Research Foundation (D.A.W.), and Jamie Swain/Voya (D.A.W.).
Authorship
Contribution: D.A.W. wrote the manuscript.
Conflict-of-interest disclosure: D.A.W. has a patent application pending as a co-inventor entitled, “Platelet Targeted Treatment” (US provisional patent application no. 61/717 951; international patent application no. PCT/US2013/066651).
Correspondence: David A. Wilcox, Department of Pediatrics, Medical College of Wisconsin, MFRC #6014, 8701 Watertown Plank Rd, Milwaukee, WI 53226; e-mail: dwilcox@mcw.edu.