In this issue of Blood, Lykken et al used an immunocompetent mouse model of B-cell lymphoma to discover an interesting new way in which these malignant cells can avoid being killed in the presence of anti-CD20 antibodies.1
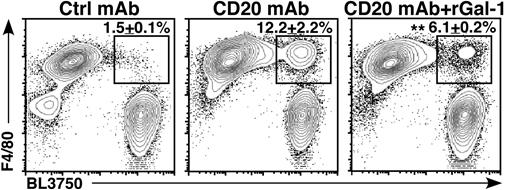
Flow cytometry analysis of phagocytosis by F4/80-positive murine peritoneal macrophages cocultured with BL3750 lymphoma cells previously incubated with anti-CD20 mAb shows addition of Gal-1 inhibits engulfment as evidenced by decreased percentage of events in the upper right quadrant. Ctrl, control; rGal-1, recombinant Gal-1. See the complete Figure 6 in the article by Lykken et al that begins on page 1886.
Flow cytometry analysis of phagocytosis by F4/80-positive murine peritoneal macrophages cocultured with BL3750 lymphoma cells previously incubated with anti-CD20 mAb shows addition of Gal-1 inhibits engulfment as evidenced by decreased percentage of events in the upper right quadrant. Ctrl, control; rGal-1, recombinant Gal-1. See the complete Figure 6 in the article by Lykken et al that begins on page 1886.
Loss of responsiveness to treatment with monoclonal antibodies (mAbs) such as rituximab is a serious complication during therapy of B-cell malignancies2 but the mechanisms responsible for it are not well understood. To promote the selection of cells refractory to antibody treatment, Lykken et al transplanted lymphoma cells into mice and shortly after injected a long-lived anti-CD20 antibody. The treatment cured a percentage of mice. Mice that nonetheless developed lymphomas were used for serial transplants and retreatment with the monoclonal. One of the mechanisms thought to be responsible for lack of responsiveness to rituximab treatment in people is loss of CD20 expression on lymphoma cells. Here, remarkably, the authors found no correlation between the expression of CD20 and whether the mice were cured or not. After comparing global gene expression in the CD20-sensitive and -resistant lymphomas, they focused their attention on galectin-1 (Gal-1) as a possible suspect for promoting resistance to the antibody because resistant tumors had increased expression of its messenger RNA.
Natural killer cells, neutrophils, and macrophages all express Fcγ receptors and can be alerted to kill abnormal cells coated with immunoglobulin G (IgG) antibodies. There is increasing evidence that macrophages can be key players in the antitumor effects exerted by some mAbs.3 Although macrophages can also directly kill their targets, a major mechanism is through antibody-dependent phagocytosis in which the opsonized target is engulfed and destroyed. This mechanism has also been reported for anti-CD20 mAbs.
How exactly does Gal-1 protect B lymphoma cells against destruction? When Lykken et al added Gal-1 to lymphoma cells coated with anti-CD20 antibodies, macrophages largely lost their ability to phagocytose them (see figure). Moreover, lymphomas engineered to overexpress Gal-1 had increased resistance to CD20 antibody therapy in mice. The mechanism through which this could operate was not explored in this study.
Gal-1 is an immune modulator with a bewildering array of activities and locations, of which the secreted form is likely to be relevant here. Gal-1 is only 14 kDa and consists of a β-galactoside–binding carbohydrate recognition domain that can latch onto glycoproteins and a protein-protein interaction domain that, through the formation of Gal-1 dimers, draws glycoprotein targets together into lattices on the cell surface. Although externally added Gal-1 can inhibit phagocytosis of IgG-coated sheep red blood cells by human macrophage,4 the mechanism of macrophage-induced killing of tumor cells has not been completely elucidated and is possibly different.5 The study also showed that inhibition of phagocytosis of the opsonized sheep red blood cells by Gal-1 is carbohydrate dependent,4 which raises the question of which glycoprotein(s) on the macrophage and possibly lymphoma cell surface could be bound by Gal-1 and inhibit lymphoma engulfment in the Lykken et al study.
Phagocytosis involves clustering of receptors for IgG at the site of opsonized target contact. Because the Fc part of IgG antibodies carries complex type N-glycans and the glycosylation of the human FcγR is varied, Gal-1 could interfere with phagocytosis by inhibition of movement of such receptors into microdomains. However, there are other possible mechanisms. For example, CD47 is a marker of “self” that prevents phagocytosis of cells expressing it by engaging with the transmembrane glycoprotein signal regulatory protein α (SIRPα) expressed on macrophages.6 Because the clustering of CD47 and SIRPα in lipid rafts on their respective cells results in the inhibition of apoptosis of the target cell, one could envision Gal-1 enhancing interactions between these proteins and augmenting the ensuing “don’t-eat-me” signal.
Although the results of Lykken et al report on the activity of a potentially important immunomodulatory protein and suggest a possible way to improve the success of anti-CD20 antibody treatment, their findings in the mouse model can most probably not be translated directly into a therapy for human patients. The model used in their studies differs from the situation in human patients as the lymphomas grew out from subcutaneously implanted tumor cells. Also, Lykken et al administered only 1 dose of antibodies a few days after tumor cell injection when there were still relatively few lymphoma cells.
To reduce the immunosuppressive effects of extracellular Gal-1 in patients treated with rituximab, therapies would need to “neutralize” Gal-1. Unfortunately, levels of Gal-1 are increased in lymphoid malignancies7 which would make this difficult to achieve. Apart from its level of abundance, it is also not easy to neutralize the protein, as it lacks enzymatic activity that could be targeted. However, antibodies against Gal-1 with an effect in preclinical mouse models have been reported.8 Perhaps the most intriguing of possible therapies are small-molecule inhibitors that dock into the carbohydrate-binding domain9 or inhibit the carbohydrate binding of Gal-1 in an allosteric manner.10 Therefore, by showing that Gal-1 inhibits phagocytosis of opsonized tumor cells, the studies by Lykken et al set the stage for further studies that explore the mechanisms behind this and how this information can be used to improve antibody therapy.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal