Abstract
Systemic immunoglobulin light chain (LC) amyloidosis (AL) is a potentially fatal disease caused by immunoglobulin LC produced by clonal plasma cells. These LC form both toxic oligomers and amyloid deposits disrupting vital organ function. Despite reduction of LC by chemotherapy, the restoration of organ function is highly variable and often incomplete. Organ damage remains the major source of mortality and morbidity in AL. This review focuses on the challenges posed by emerging therapies that may limit the toxicity of LC and improve organ function by accelerating the resorption of amyloid deposits.
Introduction
Systemic immunoglobulin light chain (LC) amyloidosis (AL) is a life-threatening condition characterized by clonal plasma cell (PC) production of immunoglobulin LCs that misfold, are toxic, and form amyloid deposits causing organ failure.1 Diagnosed in 5 to 10 persons per million in the United States annually,2 AL damages the heart, kidneys, liver, gastrointestinal tract, and peripheral nervous system. Advanced cardiac involvement at diagnosis is often fatal.3 Standard treatments seek to reduce production of LCs, using chemotherapy against clonal PCs,4 with variable rates of organ responses. Therapies beyond the PC are necessary to improve outcomes. In this review, we focus on promising emerging therapies and the challenges they pose for future research.
Clonal PCs and light chains in AL
Clonal PC biology in AL has 3 key features: λ to κ frequency of 4:1, restricted LC variable region germ line gene use, and a genetic abnormality that explains the predominance of LC-producing clones.5-10 Moreover, the clonal PC burden at diagnosis influences AL organ tropism6 and clinical outcomes.5,11 PC genetics are also relevant12 : About 25% of patients with AL have clones containing gain 1q, whereas 35% have monosomy 13 and 60% t(11;14).12-14 The t(11;14) translocation, deleting the heavy chain locus, likely accounts for the high frequency of LC-producing clones. Cytogenetic and fluorescence in situ hybridization abnormalities in AL PCs may also influence hematologic and organ responses.12-16
Certain λ LC germ line genes display organ tropism6,9,17,18 ; clones using IGVL6-57 are associated with renal involvement, whereas those using IGVL1-44 are associated with cardiac involvement.6,18 The restricted repertoire of LC variable region germ line gene use may also contribute to the presence of generic epitopes that antiamyloid antibodies target in misfolded LC and fibrillar deposits.19
The pathologic mechanisms of symptomatic disease include both toxicity of LC and mass effects of deposits, both modulated by LC concentration and physicochemical features of the misfolded LCs that remain poorly understood (“amyloidogenicity”). Despite the variability observed in amyloid accumulation within the organs of a patient, as well as among patients with the same type of amyloidosis,20 the fibrillar deposits contain a unique proteomic signature of chaperone proteins, including serum amyloid P (SAP, a pentraxin that protects fibrils from resorption) protein, vimentin, vitronectin, and apolipoprotein E.21-23 Turnover of deposits occurs by macrophage-related processes that can lead to significant resorption if production of the culprit amyloid-forming protein is stopped20 ; hence, some patients with AL improve significantly with elimination of clonal LCs.24
Cardiac amyloidosis is the priority
Although survival has improved significantly during the past 2 decades,8 cardiac involvement, not PC genetics, remains the primary cause of death. The use of cardiac biomarkers (N-terminal pro-brain natriuretic peptide [NT-proBNP] and cardiac troponins) to stage cardiac involvement has had a major effect on the management of AL.25 The cardiotoxicity of amyloid-forming LC involves activation of stress-related signaling cascades that upregulate expression of the NT-proBNP gene NPPB.26-28 Mayo stages III and IV have median survivals of 14 and 5.8 months, respectively,29 and despite treatment with proteasome inhibitor-based frontline therapy, only 63% of patients with advanced cardiac involvement are alive at 2 years.3 Chemotherapy can reduce the toxic effects of LC by rapidly decreasing LC concentration, but sudden deaths still occur.
Hematologic response is not sufficient for organ improvement
Modern response criteria based on the free LC assay and the cardiac biomarker NT-proBNP provide surrogates for time-to-event endpoints,30,31 supplementing earlier criteria.32 The recognition that hematologic response is necessary for organ response is being eclipsed, however, by the recognition that it is not sufficient for organ response and that both early cardiac death and survival with chronic organ damage are major unmet clinical needs. Furthermore, we do not understand the basis for reversal of AL organ damage. In a report on 313 patients with AL who were treated and achieved normalization of the free LC ratio, a surrogate for CR, organ responses were associated by univariate analysis with a difference between involved and uninvolved free LC of greater than 180 mg/L, the presence of monosomy 13 in clonal PC, the involvement of more than 1 organ at diagnosis, and baseline cardiac stage II or III.33 Other variables that may influence organ response include features of the chaperone proteins in the deposits (polymorphisms) and organ- and cell-specific processes related to genetic differences in proteolysis and phagocytosis. Why some patients with hematologic responses fail to have organ responses merits further study.
Standard therapies for AL achieve varying rates of hematologic and organ responses. Five large, independent studies have established that an NT-proBNP response is a critical predictor of survival, independent of the type of therapy.34-37 In 377 patients with AL with cardiac involvement,36 NT-proBNP responses were observed in 57% with a CR, 37% with a very good partial remission, 18% with a partial remission, and 4% of nonresponders. In a landmark analysis 6 months after starting treatment, NT-proBNP responses were observed in 26% and progression in 45% of patients, and at 2 years, 85% of responders and 30% of progressors survived. Autologous stem cell transplantation (SCT) is feasible in 25% of patients with a treatment-related mortality of 5% to 13%.38-40 In 421 patients undergoing SCT, 34% achieved CR at 1 year, 79% of whom experienced organ responses.39 Adding bortezomib and dexamethasone as consolidation after SCT resulted in CR in 58% of patients at 1 year and organ responses in 70% of patients at 2 years.41 Bortezomib and dexamethasone induction for 2 cycles followed by bortezomib-high-dose melphalan conditioning for SCT resulted in a 49% hematologic CR with renal and cardiac responses at 1 year of 35% and 57%, respectively.42 In a small, randomized trial comparing bortezomib-dexamethasone induction for 2 cycles followed by high-dose melphalan SCT to high-dose melphalan and SCT alone, the bortezomib group achieved superior hematologic CR (67.9% vs 35.7%), as well as renal responses (65.2% vs 39.1%) and cardiac responses (67% vs 25%) at 1 year.43 In patients ineligible for SCT treated with oral melphalan and dexamethasone, CR was achieved in 37% and cardiac and renal responses in 37% and 24%.44 In an intention-to-treat analysis of 230 patients receiving frontline cyclophosphamide, bortezomib, and dexamethasone, a promising regimen,45,46 the CR rate was 24%, and cardiac and renal responses occurred in 17% and 25% of the patients, respectively.47 Cross-trial comparisons of hematologic and organ responses in AL amyloidosis are inherently difficult because of the heterogeneity of patient populations, small numbers of patients in individual trials, and different analytic methods (landmark vs intention to treat). For example, there may appear to be lower organ responses when comparing similar hematologic complete response rates between SCT and conventional chemotherapy; however, patients who are eligible for SCT are highly selected patients with favorable prognostic features compared with conventional chemotherapy. Although SCT remains an effective therapy for a minority of patients and accounts for most of those who have long-term survival and organ improvement, those who survive initial therapy without achieving CR have persistent and often progressive organ damage and shortened survival, as the NT-proBNP response data indicate.
Antiamyloid therapies
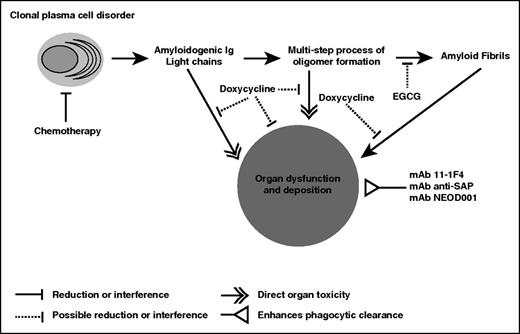
The concept of both reducing the amyloid-forming protein and accelerating resorption of deposits is gaining traction because of the novel antiamyloid therapies in development for AL and other types of systemic amyloidosis. Eprodisate, for example, has been shown to improve outcomes for patients with renal amyloid resulting from serum amyloid A, an acute-phase apolipoprotein.48 We highlight the putative mechanisms of action (Figure 1) and ongoing clinical trials (Table 1).49-56
Emerging antiamyloid agents and their putative mechanisms of action. The mechanisms of action of the antiamyloid mAb therapies currently in clinical trials are based on enhancing phagocytic clearance of amyloid deposits. Whether the removal of the deposits will enable durable organ recovery and prolong survival or time to organ failure is unknown. Doxycycline and EGCG are antiamyloid agents whose detailed mechanisms of action are not as well understood, given the limited amount of data from experimental models. The key points remain that antiamyloid therapies must be shown to provide measurable benefit to patients and are likely to be more effective when the production of the amyloid-forming protein is stopped.
Emerging antiamyloid agents and their putative mechanisms of action. The mechanisms of action of the antiamyloid mAb therapies currently in clinical trials are based on enhancing phagocytic clearance of amyloid deposits. Whether the removal of the deposits will enable durable organ recovery and prolong survival or time to organ failure is unknown. Doxycycline and EGCG are antiamyloid agents whose detailed mechanisms of action are not as well understood, given the limited amount of data from experimental models. The key points remain that antiamyloid therapies must be shown to provide measurable benefit to patients and are likely to be more effective when the production of the amyloid-forming protein is stopped.
Novel antiamyloid agents in clinical trials for AL
| Agent . | Active clinical trials . | Study population . | Key endpoints . |
|---|---|---|---|
| EGCG | Randomized phase 2 | Cardiac involvement | Change in left ventricular mass at 12 mo |
| NCT02015312 | ≥ very good partial remission before therapy | ||
| EGCG | Randomized phase 2 | Cardiac involvement | Cardiac response by NT-proBNP at 6 mo |
| NCT01511263 | > partial remission before therapy | ||
| Doxycycline | Phase 2 | Newly diagnosed AL with vital organ (heart, kidney, liver) involvement | Amyloid organ response at 1 y |
| NCT02207556 | |||
| mAb 11-1F4 | Phase 1 | Relapsed refractory AL with measurable localized or vital organ involvement | Establish safety, maximum tolerated dose |
| NCT02245867 | Assess reduction in amyloid burden | ||
| mAb anti-SAP (GSK2398852) | Phase 1* | Systemic amyloidosis, multiple types | Establish safety and dose |
| NCT01777243 | Assess reduction in amyloid burden | ||
| mAb NEOD001 | Phase 1-2* | Previously treated AL with persistent organ damage | Establish safety and dose |
| NCT01707264 | Assess cardiac and renal responses | ||
| mAb NEOD001 | Phase 3 | Newly diagnosed untreated AL with cardiac involvement, NT-proBNP between 650 and 8500 ng/L | Time to composite of all-cause mortality or cardiac hospitalization |
| NCT02312206 | |||
| mAb NEOD001 | Randomized phase 2b | Previously treated AL with persistent cardiac dysfunction, NT-proBNP 650-5000 ng/L | Best cardiac response by NT-proBNP |
| NCT02632786 |
| Agent . | Active clinical trials . | Study population . | Key endpoints . |
|---|---|---|---|
| EGCG | Randomized phase 2 | Cardiac involvement | Change in left ventricular mass at 12 mo |
| NCT02015312 | ≥ very good partial remission before therapy | ||
| EGCG | Randomized phase 2 | Cardiac involvement | Cardiac response by NT-proBNP at 6 mo |
| NCT01511263 | > partial remission before therapy | ||
| Doxycycline | Phase 2 | Newly diagnosed AL with vital organ (heart, kidney, liver) involvement | Amyloid organ response at 1 y |
| NCT02207556 | |||
| mAb 11-1F4 | Phase 1 | Relapsed refractory AL with measurable localized or vital organ involvement | Establish safety, maximum tolerated dose |
| NCT02245867 | Assess reduction in amyloid burden | ||
| mAb anti-SAP (GSK2398852) | Phase 1* | Systemic amyloidosis, multiple types | Establish safety and dose |
| NCT01777243 | Assess reduction in amyloid burden | ||
| mAb NEOD001 | Phase 1-2* | Previously treated AL with persistent organ damage | Establish safety and dose |
| NCT01707264 | Assess cardiac and renal responses | ||
| mAb NEOD001 | Phase 3 | Newly diagnosed untreated AL with cardiac involvement, NT-proBNP between 650 and 8500 ng/L | Time to composite of all-cause mortality or cardiac hospitalization |
| NCT02312206 | |||
| mAb NEOD001 | Randomized phase 2b | Previously treated AL with persistent cardiac dysfunction, NT-proBNP 650-5000 ng/L | Best cardiac response by NT-proBNP |
| NCT02632786 |
Active, not recruiting
Epigallocatechin-3-gallate
Epigallocatechin-3-gallate (EGCG) is the main polyphenolic constituent of green tea and showed activity in a mouse model of Alzheimer’s disease.57 In a report on 11 patients with AL who ingested EGCG, improvements in New York Heart Association class, decreases in septal thickness and left ventricular mass index, and increases in ejection fraction were documented.49 It is important to note that EGCG may possibly reduce the efficacy of proteasome inhibitor therapy.58,59
Doxycycline
In mice with transthyretin amyloidosis, doxycycline caused fibril disruption,60 and in a transgenic model of AL, it reduced gastric amyloid deposits.61 In a Caenorhabditis elegans model, tetracycline restored nematode pumping function damaged by LCs from cardiac patients with AL.62 In a small case-control study of bortezomib-based chemotherapy with (n = 30) or without (n = 70) doxycycline,63 NT-proBNP responses were seen in 60% of doxycycline-treated patients and 18% of controls; 82% of patients receiving doxycycline were alive at 1 year compared with 53% of controls. Retrospectively, in patients with AL undergoing SCT, doxycycline had been given as post-SCT prophylaxis to those with penicillin allergy (106 of 461 total).52 Among patients with a hematologic response, median overall survival was not reached with doxycycline compared with 161 months with penicillin. These investigations suggest the tetracyclines may reduce amyloid deposits, control LC toxicity, and have measurable clinical benefit.
Monoclonal antibodies targeting amyloid
Clinical trials testing novel antiamyloid therapies include 3 approaches based on monoclonal antibodies (mAbs): 11-1F4, anti-SAP, and NEOD001.55,64-66 All may enhance phagocyte mediated disruption and clearance of amyloid deposits. In all types of systemic amyloidosis, reduction of the amyloid-forming protein is likely also required to optimize resorption of deposits and clinical improvement.
11-1F4
The antiamyloid 11-1F4 mAb was generated by immunizing mice with a denatured human κ LC.67 11-1F4 binds to an epitope in denatured LC of both isotypes and in AL fibrils.67 In mice with flank human AL amyloidomas, 11-1F4 elicited a phagocytic response that led to elimination of the amyloidomas without apparent toxicity.68 In a phase 1 trial, I124-labeled 11-1F4 was used in 18 patients with AL, and imaging by positron emission tomography/computed tomography scan showed uptake in liver and other sites, but not in kidneys or heart.55 In a subsequent and ongoing phase 1 dose-escalation clinical trial in patients with AL with relapsed refractory disease, employing a chimeric 11-1F4 mAb (NCT02245867), cardiac and gastrointestinal organ responses were reported in 3 of the first 6 patients treated, which was a promising result.69
Anti-SAP
Anti-SAP mAb binds to SAP, a protein found in deposits and in the blood. Scans for amyloid using iodinated SAP have been used for decades in the United Kingdom.70 In the phase I anti-SAP trial, patients received 1 cycle of therapy with iodinated-SAP scans at baseline and at 6 weeks.56 To deplete circulating SAP from the blood, a depleting agent was infused, and then anti-SAP was given with no serious adverse events. Four of 8 patients had major reductions in hepatic amyloid, as well as decreases in C3 and increases in CRP, suggesting inflammatory responses.71 Importantly, there was an apparent reduction in amyloid burden in patients who had active clonal disease, including a decrease in size of an amyloid laden lymph node from 5 to 1 cm.56 Cardiac patients were excluded from the trial.
NEOD001
NEOD001 mAb reacts strongly with both κ and λ human amyloid, binding to an epitope in protofilaments and fibrils.66 In a phase 1/2 study of patients with AL in hematologic remission, but with persistent organ dysfunction, NEOD001 was infused monthly without toxicities. Cardiac and renal responses were seen in 57% and 60% of patients, including some who were years from initial therapy.54
Challenges in designing studies of agents targeting organ damage
The preclinical and clinical studies indicate that antiamyloid agents may affect outcomes in AL. However, assessing the effect of these agents in clinical trials presents numerous challenges. The most fundamental challenge is selecting the optimal endpoint for clinical trials. Because cardiac amyloidosis is the priority, endpoints that measure the effect on cardiac outcomes are essential. Studies have shown that NT-proBNP responses are a robust biomarker for outcomes in AL.31 The use of response criteria based on NT-proBNP would shorten timelines, thereby saving patients and resources in a rare disease with a significant unmet need. A limitation of NT-proBNP response assessment is selecting optimal timing for assessment, given that hematologic and organ responses are out of phase. Regulatory authorities may view NT-proBNP response as a surrogate endpoint and also employ other options for cardiac endpoints, such as patient-related outcomes and 6-minute walk test (a common endpoint in heart failure studies).
There are several possible risks to the use of antiamyloid agents. These agents could lead to a NT-proBNP “flare” confounding response assessment. Combining antiamyloid and anti-PC therapies, particularly simultaneously in the newly diagnosed setting, may result in both rapid reduction in cardiotoxic light chains and rapid amyloid resorption that could increase the risk for decompensated heart failure, arrhythmias, and sudden death. It is also possible the concomitant anti-PC chemotherapy could impair the amyloid resorption effects of antiamyloid agents. The optimal manner to integrate antiamyloid agents, either in combination or before or after chemotherapy, remains unknown. Last, we do not know whether amyloid deposits may reaccumulate more aggressively at hematologic relapse in organs that have been cleansed by these agents.
The heterogeneity of noncardiac organ involvement may affect certain endpoints. For instance, autonomic dysfunction can lead to cardiovascular events, aggressive diuresis can decrease the NT-proBNP, and peripheral neuropathy may affect the results of the 6-minute walk test. Reduction in amyloid burden in the liver can be measured by imaging and alkaline phosphatase, but may be confounded by liver regeneration or heart failure. Reversal of significant long-term proteinuria may lower the risk for progression to end-stage renal disease, thus making time to dialysis an attractive, albeit delayed, endpoint.72
Using imaging modalities to measure tissue amyloid burden and its functional consequences will be essential in clinical trials of these novel therapies. However, the optimal imaging modalities for cardiac and noncardiac organ involvement remain unknown. The addition of global longitudinal strain to standard echocardiographic parameters may prove more sensitive to the effect of these agents.73 Cardiac magnetic resonance imaging can provide quantitative measurements of amyloid burden by measuring extracellular volume fraction and left ventricular mass and is emerging as a useful tool in amyloidosis.74,75 There are several radiotracers available that can detect amyloid deposits in various organs, but there is no single radiotracer that can image the entire body. In the United Kingdom, radiolabeled SAP scintigraphy has been used extensively to image amyloid burden (aside from heart and nerve) and has been shown to be useful in the first-in-human trial of the anti-SAP antibody.56,76 As mentioned earlier, 11-1F4 conjugated to 124I can image noncardiac amyloid.55 The most promising is florbetapir, which has recently been approved by the US Food and Drug Administration for imaging Alzheimer’s disease.77 Initial studies have demonstrated that florbetapir binds to myocardial LC amyloid deposits and can detect cardiac deposits, using cardiac positron emission tomography.78,79 As so much is unknown about these modalities, intense imaging studies will need to be integrated into trials of antiamyloid agents to understand the optimal timing and ideal modalities and how specific parameters correlate with outcome. It is most likely that a combination of imaging modalities will be complementary in assessing both amyloid burden and its functional consequences.
Conclusions
Emerging therapies targeting organ involvement may provide major benefit to patients with AL. To move forward, we must develop collaborative research networks and multidisciplinary teams and recognize the limits AL imposes on clinical research with respect to sample size and accrual timelines. The constant emphasis must be on measurable clinical benefit to patients. The use of surrogate biomarkers and rational correlative studies will help us understand how, when, why, and for whom these agents may be most beneficial.
Acknowledgments
At Tufts we are grateful for the support of the John C. Davis Myeloma and Amyloid Program, the Amyloidosis and Myeloma Research Fund, the Sidewater Family Fund, the Werner and Elaine Dannheiser Fund for Research on the Biology of Aging of the Lymphoma Foundation, the Demarest Lloyd Jr Foundation, and Barbara and David Levine in memoriam.
Authorship
Contribution: B.M.W. conceived and wrote the first draft of the manuscript; S.W.W. and R.L.C. cowrote and revised subsequent revisions; and all authors agreed on the final version.
Conflict-of-interest disclosure: B.M.W. has received research support from Prothena and Janssen Research and Development and received consulting fees from Prothena and Janssen Research and Development. R.L.C. has received research support from Prothena, Takeda Millenium, Teva, and Janssen and consulting fees from Prothena, Takeda Millenium, GlaxoSmithKline, and Janssen. S.W.W. declares no competing financial interests.
Correspondence: Brendan M. Weiss, Perelman Center for Advanced Medicine, 2 West, Abramson Cancer Center, 3400 Civic Center Blvd, Philadelphia, PA 19104; e-mail: brendan.weiss@uphs.upenn.edu.