Abstract
Severe Plasmodium falciparum malaria remains a leading cause of mortality, particularly in sub-Saharan Africa where it accounts for up to 1 million deaths per annum. In spite of the significant mortality and morbidity associated with cerebral malaria (CM), the molecular mechanisms involved in the pathophysiology of severe malaria remain surprisingly poorly understood. Previous studies have demonstrated that sequestration of P falciparum–infected erythrocytes within the microvasculature of the brain plays a key role in the development of CM. In addition, there is convincing evidence that both endothelial cell activation and platelets play critical roles in the modulating the pathogenesis of severe P falciparum malaria. In this review, we provide an overview of recent studies that have identified novel roles through which hemostatic dysfunction may directly influence malaria pathogenesis. In particular, we focus on emerging data suggesting that von Willebrand factor, coagulation cascade activation, and dysfunction of the protein C pathway may be of specific importance in this context. These collective insights underscore a growing appreciation of the important, but poorly understood, role of hemostatic dysfunction in malaria progression and, importantly, illuminate potential approaches for novel therapeutic strategies. Given that the mortality rate associated with CM remains on the order of 20% despite the availability of effective antimalarial therapy, development of adjunctive therapies that can attenuate CM progression clearly represents a major unmet need. These emerging data are thus not only of basic scientific interest, but also of direct clinical significance.
Introduction
Severe Plasmodium falciparum malaria remains a leading cause of mortality in sub-Saharan Africa, where it accounts for up to 1 million deaths per annum, particularly in children under 5 years of age.1,2 Cerebral malaria (CM), is a life-threatening complication of P falciparum that develops in ∼1% of infections and is characterized by a diffuse encephalopathy resulting in decreased consciousness and unrousable coma.3 Children typically present with ataxia, seizures, and unrousable coma. Although effective antimalarial drugs have been developed, CM is still associated with a case fatality rate of 15% to 20%.4 In addition, a significant proportion (10% to 20%) of children who survive CM suffer significant neurologic sequelae, which can include learning difficulties and memory impairment.4,5 In spite of this significant mortality and morbidity, the molecular mechanisms involved in the pathophysiology of severe malaria remain surprisingly poorly understood.
Previous studies have demonstrated that sequestration of P falciparum–infected erythrocytes (IEs) within the microvasculature of the brain plays a key role in the development of CM.6,7 This sequestration process, which involves the cytoadhesion of IEs to endothelial cell (EC) surfaces, has been extensively studied and is mediated by parasite-related ligands, such as P falciparum erythrocyte membrane protein 1 (PfEMP1), which are exported to the IE surface.8,9 In addition, a number of specific EC surface receptors have been shown to modulate the adhesion of IEs, including thrombomodulin (TM), endothelial protein C receptor (EPCR), CD36, thrombospondin, intercellular adhesion molecule-1, vascular adhesion molecule-1, P-selectin, and E-selectin.9 Importantly, expression of these EC receptors varies between different vascular beds and is subject to regulation by inflammatory cytokines (eg, tumor necrosis factor and interleukin-1).10,11 Consequently, inflammation and EC activation play critical roles in regulating the sequestration of IEs within the brain microvasculature, which is a characteristic feature of CM.7,12 Although the pathogenesis remains poorly defined, it is likely that IE sequestration leads to obstruction of brain microvessels, reduced blood flow, and ultimately cerebral hypoxia. Interestingly, postmortem studies on patients with CM have suggested that platelets and leukocytes may also be sequestered within the cerebral microvasculature and thereby contribute to vessel occlusion.7 In addition to this mechanical obstruction of the cerebral microvasculature, significant evidence suggests that proinflammatory cytokines secreted in response to the presence of IEs (including tumor necrosis factor and interferon-γ) may also be important in modulating the development of CM.13 The relative importance of IE sequestration vs cytokine secretion in the etiology of CM remains a contentious area that has been reviewed previously in detail.7,13,14 Previous studies reporting diminished vascular nitric oxide (NO) bioavailability in individuals with CM compared with healthy individuals further underscore the importance of dysregulated EC activation in CM pathogenesis15 Impaired NO production promotes enhanced EC activation and IE cytoadherence16 and is associated with increased mortality in a murine CM model.17 Furthermore, reduced vascular NO bioavailability also promotes angiopoietin-2 release from ECs, which may in turn disrupt the angiopoietin-1/Tie-2–dependent signaling axis that maintains EC quiescence and vascular integrity.18,19
In this review, we provide an overview of recent studies that have identified novel roles through which hemostatic dysfunction may directly influence P falciparum pathogenesis. In particular, we focus on specific emerging data suggesting that (1) von Willebrand factor (VWF), (2) coagulation cascade activation, and (3) dysfunction of the protein C pathway may play a role in this context. As our understanding of the biological mechanisms underpinning these observations continues to progress, it seems likely that significant opportunities for novel therapeutic strategies may arise. Given the major morbidity and mortality associated with CM, these emerging data are thus not of only of basic scientific interest, but also of direct clinical significance.
VWF in malaria
VWF is a large plasma glycoprotein synthesized within ECs and megakaryocytes.20,21 VWF circulates in normal plasma as a series of heterogeneous multimers and plays 2 essential roles in normal hemostasis.21 First, VWF mediates the adhesion of platelets to exposed collagen at sites of vascular injury.20 Second, VWF acts as a carrier molecule for procoagulant factor (F) VIII, thereby protecting it from premature proteolytic degradation and clearance.22 VWF synthesized within ECs is either constitutively secreted into the plasma or, alternatively, stored within intracellular organelles known as Weibel-Palade (WP) bodies.23,24 This stored VWF is enriched in high-molecular-weight multimers and is secreted together with other WP contents following EC activation.24 A series of recent studies have shown that plasma levels of VWF antigen (VWF:Ag) and VWF propeptide (VWFpp) are both markedly elevated in patients with severe P falciparum malaria.25-29 For example, Hollestelle et al observed that plasma VWF concentration at presentation in Ghanaian children with CM was approximately threefold higher than that observed in healthy age-matched controls.25 Interestingly, plasma VWFpp levels were also threefold increased in children with CM.25 Using the selective expression of ABO(H) blood group antigens on EC-derived VWF,25,30 Hollestelle et al demonstrated that the elevated plasma VWF:Ag levels in CM patients were predominantly derived from activated ECs by showing that in blood group A CM patients, blood group A antigen expression on VWF increased concurrently with plasma VWF:Ag. Interestingly, peak levels of plasma VWF:Ag and VWFpp levels in some children with CM were increased more than fivefold, exceeding those previously seen in fulminant vascular diseases such as thrombotic thrombocytopenic purpura.25,31
Significantly increased plasma VWF:Ag and VWFpp levels have been confirmed in a number of other independent malaria cohort studies. Although these subsequent studies recruited combinations of both adult and children from a variety of different geographic locations (including Indonesia, Bangladesh, Uganda, and Malawi), all observed markedly elevated plasma VWF:Ag and VWFpp levels (approximately three- to fivefold) in patients with severe malaria compared with healthy local controls.32,33 Although the absolute VWF:Ag levels were more modest, significantly elevated plasma VWF:Ag was also seen in an Indonesian cohort of patients with Plasmodium vivax malaria.32 Collectively, these studies clearly demonstrate that markedly elevated VWF:Ag and VWFpp levels constitute consistent features of severe malaria infection. Importantly, in a study of 14 healthy volunteers infected with P falciparum, de Mast et al further showed that the increase in plasma VWF:Ag and VWFpp develops at a very early stage following the onset of blood-stage infection, when estimated levels of IEs remain <0.001%.34 The molecular mechanisms responsible for this early EC activation and WP body secretion following P falciparum infection have not been elucidated. However, previous studies have shown that P falciparum does secrete a functional histamine release factor that may contribute to WP body exocytosis.35
As well as the marked increase in plasma VWF levels in severe malaria, a pathological accumulation of ultralarge VWF (UL-VWF) multimers has also been reported by several groups.26,32 Importantly, these UL-VWF multimers demonstrate significantly enhanced binding to platelets compared with monomers and consequently are more efficient in modulating platelet aggregation. Furthermore, a significant proportion of plasma VWF in patients with P falciparum infection circulates in an active confirmation that facilitates binding to platelet GpIb.34,36 In addition, in vitro experiments have demonstrated that platelet-decorated UL-VWF strings on the surface of activated ECs can tether P falciparum IEs under physiological shear stress.37 This interaction is modulated by PfEMP-1 on the surface of IEs binding to platelet CD36. Given that previous studies have highlighted that platelet adhesion and aggregation are important in the development of cerebral microvasculature occlusion in CM,38-40 these emerging findings regarding a putative role for VWF in malaria are of significant interest. In particular, these data raise the intriguing possibility that the early marked increase in plasma VWF levels may play a specific role in modulating malaria pathogenesis. This hypothesis is supported by observations from studies of children with severe malaria demonstrating significant correlations between VWFpp levels and other established biomarkers of malaria severity including plasma lactate.25 Furthermore, plasma VWF:Ag levels are also inversely related with both platelet count and overall survival.25,36 Interestingly, ABO blood group glycan determinants are expressed on both the N- and O-linked glycans of VWF.41,42 Moreover, plasma VWF:Ag levels are significantly reduced in blood group O compared with non-O individuals.43,44 In addition, group O VWF also demonstrates significantly enhanced susceptibility to proteolysis by ADAMTS13.43,45 Collectively, these findings are of interest because blood group O individuals are significantly protected against P falciparum malaria.46,47
Defining whether the early increase in plasma VWF levels and/or the presence of UL-VWF multimers merely constitute biomarkers of EC activation or whether VWF actually contributes to the pathogenesis of P falciparum malaria is difficult to address in human studies. However, recent studies from our laboratory using a murine model of experimental CM in which C57BL/6J mice are infected with Plasmodium berghei ANKA provide further support for the hypothesis that VWF plays a direct role in malaria pathogenesis. Following P berghei inoculation, a significant increase in murine plasma VWF:Ag levels was observed.48 Consistent with previous studies in children with CM, peak VWF:Ag levels were increased ∼2.5-fold in mice with CM compared with uninfected controls. Also in keeping with the observations of the human volunteer studies, a significant increase in murine plasma VWF:Ag levels was apparent from an early stage following P berghei infection, prior to the development of significant blood parasitemia levels. In addition, a pathological accumulation of UL-VWF was also observed in the plasma of mice inoculated with P berghei. Finally, VWF−/− mice were significantly protected against experimental CM.48 Although the molecular mechanism(s) through which VWF influences malaria progression has not been elucidated, a number of plausible mechanisms may be proposed (Figure 1). First, as previously described, in vitro studies have demonstrated that platelet-decorated UL-VWF strings can tether IEs under conditions of physiological shear stress.37 Thus, VWF may directly facilitate the cytoadhesion of IEs to EC surfaces. Second, VWF also modulates leukocyte and monocyte adhesion to ECs49,50 and also has been shown to regulate leukocyte extravasation.51 Finally, murine studies have demonstrated a role for VWF in regulating blood-brain barrier permeability,52,53 which is important because previous studies have reported that blood-brain barrier dysfunction is important in the pathogenesis underlying CM.54-56 Further studies will be required to fully understand how VWF influences malaria progression in vivo. Nonetheless, these emerging human and murine data suggest that VWF plays a novel role in malaria pathogenesis.
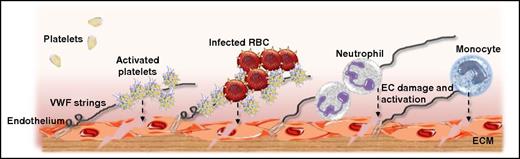
Putative mechanisms through which VWF secreted from ECs may influence the pathogenesis of P falciparum malaria. At an early stage following P falciparum infection, acute EC activation results in WP body exocytosis. Consequently, plasma VWF:Ag levels are significantly increased, and pathological UL-VWF multimers accumulate in the plasma. While tethered on the surface of activated ECs, UL-VWF can bind circulating platelets. These bound activated platelets further activate the underlying ECs (see dashed line). In addition, CD36 expressed on the platelet surface of these platelet-decorated UL-VWF strings can bind to PfEMP1, thereby facilitating the cytoadhesion and sequestration of IEs. This VWF-modulated IE cytoadhesion may be of particular importance in the cerebral microvasculature where there is no constitutive CD36 expression on ECs. Once again, IE cytoadhesion further stimulates EC activation and dysfunction. Finally, in addition to being important in modulating platelet and IE recruitment, VWF can also bind to neutrophils and monocytes, both of which can enhance EC damage resulting in enhanced EC permeability. ECM, experimental cerebral malaria; RBC, red blood cell.
Putative mechanisms through which VWF secreted from ECs may influence the pathogenesis of P falciparum malaria. At an early stage following P falciparum infection, acute EC activation results in WP body exocytosis. Consequently, plasma VWF:Ag levels are significantly increased, and pathological UL-VWF multimers accumulate in the plasma. While tethered on the surface of activated ECs, UL-VWF can bind circulating platelets. These bound activated platelets further activate the underlying ECs (see dashed line). In addition, CD36 expressed on the platelet surface of these platelet-decorated UL-VWF strings can bind to PfEMP1, thereby facilitating the cytoadhesion and sequestration of IEs. This VWF-modulated IE cytoadhesion may be of particular importance in the cerebral microvasculature where there is no constitutive CD36 expression on ECs. Once again, IE cytoadhesion further stimulates EC activation and dysfunction. Finally, in addition to being important in modulating platelet and IE recruitment, VWF can also bind to neutrophils and monocytes, both of which can enhance EC damage resulting in enhanced EC permeability. ECM, experimental cerebral malaria; RBC, red blood cell.
ADAMTS13 in malaria
UL-VWF multimers secreted from WP bodies normally undergo partial proteolysis on the EC surface by the zinc metalloproteinase ADAMTS13.57,58 ADAMTS13 cleaves at a specific peptide bond (Y1605/M1606) within the VWF A2 domain and thus prevents accumulation of UL-VWF multimers in normal plasma.59,60 The molecular mechanism(s) responsible for the presence of UL-VWF in patients with severe malaria infection has not been defined. However, several studies have reported that plasma ADAMTS13 antigen and activity levels are significantly reduced in CM.26,32,33 Interestingly, only modest reductions in ADAMTS13 have been observed, with plasma levels generally remaining >30% normal values.26,32,33 Previous in vitro studies suggest that these ADAMTS13 levels should be adequate to maintain normal plasma VWF multimer distribution.61 Consequently, the significance of this modest reduction in plasma ADAMTS13 with respect to the accumulation of UL-VWF multimers in children with severe P falciparum malaria remains unclear.
Preliminary studies have suggested that ADAMTS13 activity may also be inhibited in plasma from P falciparum–infected children.26 Although the physiological regulation of ADAMTS13 activity is not fully understood, a number of putative inhibitors have been described. These include thrombin, interleukin-6 (IL-6), thrombospondin 1, and free plasma hemoglobin.62-65 Of these, plasma IL-6 and free hemoglobin levels have both been shown to be significantly elevated in children with CM.26 However, the increases in plasma IL-6 and hemoglobin levels in severe malaria are significantly below the threshold levels necessary to significantly inhibit ADAMTS13 activity in vitro.26,62,63 In summary, it seems likely that multiple biological mechanisms (including marked acute EC activation and release of UL-VWF from WP bodies, significantly reduced plasma ADAMTS13 antigen levels, and inhibition of ADAMTS13 functional activity) contribute to the accumulation of abnormal UL-VWF multimers in patients with severe P falciparum malaria. Additional studies will be required to define the relative importance of these different mechanisms.
Coagulation cascade activation in malaria
Thrombocytopenia is a common finding in patients with P falciparum malaria.12,66 The mechanisms responsible for this thrombocytopenia have not been fully elucidated, but a number of putative mechanisms have been proposed.66,67 In addition, a series of studies dating back to the 1960s have shown that coagulation cascade activation is also common in both children and adults with P falciparum infection.68-73 Many of the older studies of coagulation factor levels in malaria included relatively small numbers and enrolled patient cohorts with variable characteristics at presentation (including differences in age ranges, ethnicity, and malaria severity). Consequently, it is perhaps not surprising that variation in results was observed between the individual studies.74 Nevertheless, these older studies in combination with more recent reports collectively support the hypothesis that P falciparum malaria is associated with significant coagulation activation.74,75 In particular, significant elevations in plasma levels of thrombin-antithrombin complexes, fibrin degradation products, and d-dimers have been consistently reported.69,70,72,73 Although mild prolongations in both the prothrombin time and activated partial thromboplastin time have been noted, plasma fibrinogen levels typically remain within the normal range.68,69,73 Interestingly, the degree of coagulation activation in malaria has been associated with disease severity and parasitemia levels in several studies.72,76 Finally, in keeping with this state of coagulation activation, significant reductions in plasma anticoagulant factors (including antithrombin, protein C, and protein S) have also been observed in patients with malaria.69-71
Moxon et al recently used the International Society for Thrombosis and Haemostasis scoring algorithm77 to objectively assess the prevalence and significance of disseminated intravascular coagulation (DIC) in a cohort of 176 Malawian children presenting with CM.73 In order to further refine the CM diagnosis, all children with CM also underwent ophthalmology review. Children with characteristic retinal changes associated with IE sequestration in the cerebral microvasculature (whitening, vessel changes and/or microhemorrhages) were defined as “retinopathy positive.” In contrast, in cases without these retinal changes (“retinopathy negative”), other etiologies for the encephalopathy were considered. Using this systematic approach, plasma levels of d-dimers, fibrin degradation products, and fibrin monomers were all significantly elevated in children with retinopathy-positive CM compared with controls.73 In addition, an International Society for Thrombosis and Haemostasis score consistent with overt DIC (≥5) was observed in 19% of children with retinopathy-positive CM. Importantly, in this subgroup of children with retinopathy-positive CM and overt DIC, overall mortality was significantly increased (odds ratio, 3.068; P = .035). Despite the fact that thrombocytopenia, coagulation activation, and DIC are common in P falciparum malaria, clinical bleeding and/or thrombotic complications are rarely observed in affected children.73,78 Although the reported incidence varies considerably between different studies, hemorrhagic complications and thrombotic sequelae do appear more common in adult patients with severe malaria.70,79 For example, Clemens et al observed significant bleeding (melena, ecchymoses, hemoptysis, and hematemsis, respectively) in 4 of 22 adult Thai patients admitted with severe P falciparum malaria.70 In addition, other studies have reported pulmonary hemorrhage80 and intracranial bleeding in adult patients with severe malaria.81 Nevertheless, even in adult patients with severe P falciparum, the overall risk of developing overt clinical bleeding or thrombotic complications has been estimated at <5%.70,79
The molecular mechanism(s) through which P falciparum infection causes in vivo coagulation activation remains poorly understood. However, Francischetti et al have previously demonstrated that incubation of IEs with mircovascular ECs in vitro results in the induction of EC tissue factor (TF) expression.82 Interestingly, this TF expression on ECs was found to be dependent on P falciparum parasite developmental stage. Consequently, although significant TF expression on ECs was observed following incubation with mature forms of parasitized red blood cells (late trophozoites and schizonts), only minimal TF was detected after incubation with early and midtrophozoite stages. Furthermore, immunohistochemical studies on postmortem samples from CM cases have also demonstrated aberrant TF expression on ECs within the cerebral microvasculature.82 In addition to the upregulation of TF expression, 2 independent previous studies have shown that P falciparum infection leads to the expression of negatively charged phophatidylserine (PS) on red cell membrane surfaces.82,83 Interestingly, when coincubated with FXa, FVa, Ca2+, and prothrombin, these IEs supported thrombin generation in vitro.82 Similarly, incubation of IEs with FVIIIa, FIXa, Ca2+, and FX also resulted in significant FXa generation. In contrast, if the IEs were replaced with normal red blood cells in these experiments, no thrombin or FXa generation was observed. A further mechanism by which P falciparum infection may promote coagulation is by expression of procoagulant protein(s) that enters the blood stream upon IE rupture and impact upon hemostasis. One such protein is the P falciparum–specific histidine-rich protein II (HRPII).84 Although the specific purpose of HRPII is not well understood, recent studies indicate that it binds glycosaminoglycans (GAGs) with exceptionally high affinity in the presence of zinc or copper ions.85 Consequently, HRPII-GAG binding significantly impedes GAG-mediated antithrombin inhibition of FXa and thrombin in plasma and reverses the anticoagulant activity of heparin.85 The ability of HRPII to potently inhibit a crucial plasma anticoagulant process implies a potential mechanism by which P falciparum–encoded proteins can directly modulate coagulation. Together, these findings suggest that P falciparum triggers coagulation activation through multiple different pathways that include induction of TF expression on microvascular ECs; the exposure of negatively charged PS on the surface of IEs, which enables assembly of the intrinsic tenase and prothrombinase complexes; and direct inhibition of key endogenous anticoagulants (Figure 2).
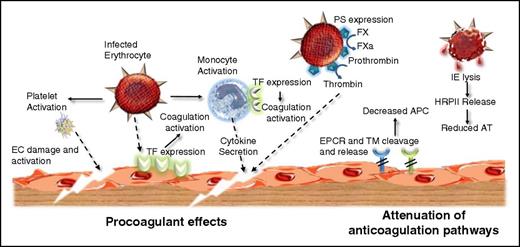
P falciparum infection causes coagulation activation through multiple different mechanisms. Procoagulant effects: P falciparum infection causes early EC activation and platelet activation. Furthermore, IEs induce TF expression on EC surfaces and on monocytes. This aberrant intravascular TF expression in combination with FVIIa results in initiation of the extrinsic coagulation pathway. In addition, P falciparum infection leads to expression of negatively charged PS on the red cell surface. This PS enables the assembly of the intrinsic tenase and prothrombinase complexes, thereby enhancing coagulation amplification. Activated clotting factor proteases (notably FXa and thrombin) generated through P falciparum–induced coagulation activation interact with specific EC surface including protease-activated receptor (PAR) 1 and thereby initiate downstream intracellular signaling, which ultimately results in enhanced EC activation, damage, and apoptosis. Attenuation of normal anticoagulant effects: In addition to the specific procoagulant effects described previously, P falciparum infection further promotes coagulation activation by downregulating normal endogenous anticoagulant pathways. EC surface expression of TM and the EPCR are both reduced, likely because of cytokine-enhanced shedding. Moreover, plasma levels of soluble TM and soluble EPCR (sEPCR) are both increased. Together, these effects combine to lead to a significant reduction in generation of anti-inflammatory and cytoprotective activated protein C (APC) on the EC surface. Finally, release of HRPII following spontaneous IE lysis significantly inhibits the anticoagulant effects of antithrombin (AT).
P falciparum infection causes coagulation activation through multiple different mechanisms. Procoagulant effects: P falciparum infection causes early EC activation and platelet activation. Furthermore, IEs induce TF expression on EC surfaces and on monocytes. This aberrant intravascular TF expression in combination with FVIIa results in initiation of the extrinsic coagulation pathway. In addition, P falciparum infection leads to expression of negatively charged PS on the red cell surface. This PS enables the assembly of the intrinsic tenase and prothrombinase complexes, thereby enhancing coagulation amplification. Activated clotting factor proteases (notably FXa and thrombin) generated through P falciparum–induced coagulation activation interact with specific EC surface including protease-activated receptor (PAR) 1 and thereby initiate downstream intracellular signaling, which ultimately results in enhanced EC activation, damage, and apoptosis. Attenuation of normal anticoagulant effects: In addition to the specific procoagulant effects described previously, P falciparum infection further promotes coagulation activation by downregulating normal endogenous anticoagulant pathways. EC surface expression of TM and the EPCR are both reduced, likely because of cytokine-enhanced shedding. Moreover, plasma levels of soluble TM and soluble EPCR (sEPCR) are both increased. Together, these effects combine to lead to a significant reduction in generation of anti-inflammatory and cytoprotective activated protein C (APC) on the EC surface. Finally, release of HRPII following spontaneous IE lysis significantly inhibits the anticoagulant effects of antithrombin (AT).
The significant coagulation activation and DIC observed in patients with severe P falciparum has led to suggestions that hemostasis dysfunction may play a pivotal role in malaria pathogenesis.75,86,87 This hypothesis is supported by a number of lines of evidence. In particular, postmortem studies in both children and adults with fatal CM have demonstrated the presence of fibrin within the microvasculature.74,87 In addition, the degree of hemostatic dysfunction observed in patients with malaria has been reported to correlate with disease severity and peripheral blood parasitemia levels.72,76 However, it is important to note that other studies failed to demonstrate significant fibrin deposition in CM cases at postmortem.74 Consequently, analogous to the previous discussion regarding a putative role for VWF in malaria, it remains unclear whether coagulation cascade activation is directly involved in modulating the pathogenesis of P falciparum malaria, or whether instead this coagulation activation merely represents a secondary epiphenomenon. Although previous studies demonstrated no beneficial effect with heparin anticoagulation in patients with severe malaria,88 further studies will be necessary to determine whether other targeted coagulation cascade inhibition (eg, specific FXa or FIIa inhibition with direct oral anticoagulant agents) may have a role to play.
Malaria and the protein C pathway
The protein C pathway is a crucial anticoagulant and anti-inflammatory pathway triggered in response to the generation of excess thrombin production during clot formation.89 Thrombin engages TM on intact endothelium, where its substrate specificity is switched to favor activation of protein C rather than procoagulant substrates that include FV, FVIII, FXI, and fibrinogen. Protein C activation by the thrombin-TM complex is accelerated by the presence of EPCR, which binds protein C with high affinity and presents it for optimal activation by the thrombin-TM complex.90 APC, in conjunction with its cofactor protein S, then restricts further thrombin generation by proteolytic degradation of activated cofactors V and VIII. Further to its anticoagulant activity, APC can also trigger myriad cell-signaling pathways via cell surface activation of protease-activated receptor (PAR) 1, PAR3, apolipoprotein E receptor 2, Tie2, and integrin αMβ2.89 Cell signaling by APC, although diverse, is united by its ability to initiate protective cellular responses upon exposure to proinflammatory, proapoptotic, or toxic insult.91
Previous studies have demonstrated that plasma protein C levels are significantly reduced in retinopathy-positive CM compared with individuals with nonsevere malaria or nonmalaria induced coma.73 Moreover, recent studies suggest vascular protein C pathway receptors can also bind PfEMP1 and thereby enable the cytoadhesion and sequestration of IEs.92 In addition to early reports that suggested PfEMP1 can bind TM via chondroitin sulfate moieties expressed near the TM membrane proximal region,93 EPCR has recently been described as a dominant target for PfEMP1 subtypes associated with severe malaria.92 Specifically, N-terminal cysteine-rich interdomain region (CIDR) domains α1.1 and α1.4-1.8 that form part of PfEMP1 bind to EPCR with high affinity (Figure 3). Interestingly, individuals living in areas where malaria transmission is high have been reported to develop antibodies against the EPCR-binding CIDRα1 domain subtypes early in life, which implies a key role for antibody generation against EPCR-binding CIDRα1 domains in generating effective immunity to severe malarial disease.94
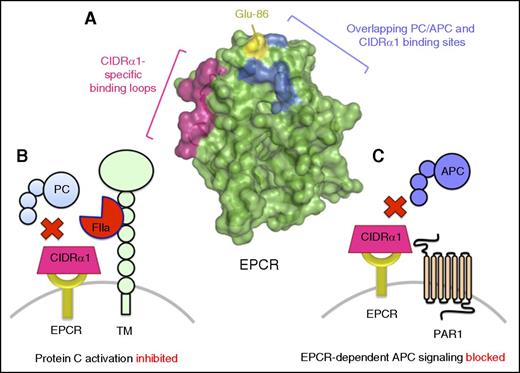
EPCR-binding PfEMP subtypes use CIDRα1 domains with overlapping EPCR-binding sites to protein C/APC to restrict protein C pathway function. (A) PfEMP subtypes expressing domain cassettes DC8 and DC13 that include CIDRα1.1 and CIDRα1.4-1.8 domains bind EPCR with high affinity. EPCR-binding CIDRα1 domains appear to use a similar binding mechanism and binding site to that of protein C/APC (blue). As CIDRα1 domains are significantly larger than the EPCR-binding region of protein C/APC, EPCR-binding CIDRα1 domains make additional extended contacts with EPCR via loops containing amino acid residues 22 to 25 and 44 to 47 (pink). However, despite the significant overlap, amino acid residues that include Glu-86 (yellow) have been identified that are crucial for protein C/APC binding, but not PfEMP1. Accordingly, recombinant variants of sEPCR in which Glu-86 has been substituted have been proposed as a potential therapeutic strategy to competitively impede EPCR-binding PfEMP subtypes enabling IE cytoadherence to the vasculature, preventing blockade of EPCR-dependent protein C activation (B) and PAR1 cytoprotective signaling by APC (C).
EPCR-binding PfEMP subtypes use CIDRα1 domains with overlapping EPCR-binding sites to protein C/APC to restrict protein C pathway function. (A) PfEMP subtypes expressing domain cassettes DC8 and DC13 that include CIDRα1.1 and CIDRα1.4-1.8 domains bind EPCR with high affinity. EPCR-binding CIDRα1 domains appear to use a similar binding mechanism and binding site to that of protein C/APC (blue). As CIDRα1 domains are significantly larger than the EPCR-binding region of protein C/APC, EPCR-binding CIDRα1 domains make additional extended contacts with EPCR via loops containing amino acid residues 22 to 25 and 44 to 47 (pink). However, despite the significant overlap, amino acid residues that include Glu-86 (yellow) have been identified that are crucial for protein C/APC binding, but not PfEMP1. Accordingly, recombinant variants of sEPCR in which Glu-86 has been substituted have been proposed as a potential therapeutic strategy to competitively impede EPCR-binding PfEMP subtypes enabling IE cytoadherence to the vasculature, preventing blockade of EPCR-dependent protein C activation (B) and PAR1 cytoprotective signaling by APC (C).
Recent elegant crystallography studies of 2 sEPCR-bound PfEMP1 CIDRα1 domains (HB3var03 and IT4var07) have revealed the key structural requirements for PfEMP1 binding to EPCR.95 Interestingly, both CIDRα1 domains bind to sEPCR using a binding site that overlaps significantly with that of protein C/APC. Moreover, despite the considerable sequence diversity exhibited by the EPCR-binding regions of each tested CIDRα1 domain, core structural features enabling hydrophobic interactions are retained, such that the sequence diversity that characterizes PfEMP1 does not impact negatively upon EPCR binding. Additional studies have suggested that other CIDRα1.1 and CIDRα1.4 domains, not previously characterized by crystallography, can also bind EPCR in subtly different conformations.96 Collectively, therefore, CIDRα1 domains share a largely conserved EPCR-binding mechanism, but sequence-specific differences between PfEMP1 subtypes may confer subtle modifications to binding site and affinity with the potential to mediate divergent effects on EPCR function.97
PfEMP binding to EPCR appears to contribute to malaria pathogenesis beyond IE cytoadherence. Recent in vitro studies indicate that PfEMP binding to EPCR limits protein C activation, EPCR-dependent PAR1 activation, and PAR1-dependent protection of the EC barrier integrity.97,98 Consequently, PfEMP1 binding would be predicted to severely attenuate normal protein C pathway activity during P falciparum infection. In addition, the significant proinflammatory response associated with severe malaria is likely to induce further protein C pathway dysfunction. sEPCR and soluble TM, presumably cleaved from the inflamed vascular surface, are significantly elevated in the cerebrospinal fluid of CM patients compared with nonmalarial febrile patients.87 Moreover, reduced expression of both receptors has been reported in the subcutaneous microvasculature from individuals with severe P falciparum malaria at sites of local IE cytoadherence.87 As observed in other inflammatory disease settings, diminished EC surface expression of EPCR and TM acts as a brake on the protein C pathway in responding to proinflammatory stimuli, leading to unregulated thrombin generation, local fibrin deposition, and defective vascular anti-inflammatory activity. In this way, competitive blockade of EPCR availability to drive protein C activation and promote PAR1-dependent anti-inflammatory activity amplifies further protein C pathway dsyfunction to enable further P falciparum–mediated vascular damage.
Despite the development of effective antimalarial therapies, CM is still associated with significant mortality. Consequently, adjunctive therapies to limit vascular dysfunction and slow disease progression are urgently required. In this setting, 1 attractive therapeutic target would be to use the dual anticoagulant and anti-inflammatory properties of APC. Recombinant APC has previously been used in the treatment of severe sepsis99 and is currently under evaluation to enhance the safety of thrombolytic stroke therapy.100 Given the thrombocytopenia and DIC associated with severe P falciparum malaria, use of wild-type (WT) APC may be associated with significant bleeding risk. Nevertheless, some cases studies have reported beneficial effects following the use of WT APC infusion in patients with severe malaria.101 Alternatively, signaling-selective engineered APC analogs with diminished anticoagulant properties may offer a safer approach.102 An alternative potential therapeutic strategy is to use the high affinity of EPCR for PfEMP1 CIDRα1 domains to create recombinant sEPCR analogs that could prevent IE cytoadherence to the vessel wall. To this end, a bioengineered sEPCR analog containing an amino acid substitution (E86A) that has previously been demonstrated to ablate protein C binding was developed.98 Importantly, this E86A substitution does not attenuate the ability of EPCR to bind PfEMP1. Unlike WT sEPCR, sEPCR-E86A did not influence normal protein C function or APC anticoagulant and signaling activity but bound to recombinant CIDRα1 domains with similar affinity to WT sEPCR,98 suggesting a possible means by which this PfEMP1 cytoadherence strategy could be therapeutically targeted, without deleterious impact on normal protein C pathway function.
Four EPCR haplotypes have been previously described.103 Individuals with the H3 EPCR haplotype possess the g.4600A>G allele that encodes a single amino acid substitution (S219G) within the EPCR transmembrane region. This S219G substitution in individuals with the EPCR H3 haplotypes is associated with a fourfold increase in sEPCR plasma levels.103 Recent studies have examined whether individuals with the H3 haplotype exhibit altered susceptibility to malaria. To date, studies have provided conflicting results. For example, a large Ghanaian association study failed to link EPCR H3 haplotype with malaria disease severity.104 In contrast, a recent Thai study found that elevated sEPCR plasma levels in individuals with H3 haplotype were associated with protection from CM.105 Evidently, larger studies are required to unequivocally determine whether EPCR haplotype and constitutively high sEPCR plasma concentration truly have an effect on malaria disease severity.
Conclusions
In conclusion, there is overwhelming evidence that both EC activation and platelets play critical roles in the modulating the pathogenesis underlying severe P falciparum malaria. Although the molecular mechanisms have not been fully defined, the emerging data discussed in this review support the hypothesis that specific aspects of hemostatic dysfunction may also be important in malaria progression. Further animal and human studies will be essential in order to elucidate which of these hemostatic effects are directly involved in malaria pathogenesis and which merely arise as secondary epiphenomena. In addition, the clinical utility of specifically targeting these novel molecular mechanisms with new therapeutic strategies will need to be defined. However, given that the mortality rate associated with CM remains on the order of 20% despite the availability of effective antimalarial therapy, development of adjunctive therapies that can significantly attenuate CM progression clearly represents a major unmet need.
Acknowledgments
This work was supported by the Children’s Medical and Research Foundation, Our Lady’s Children’s Hospital, Crumlin (J.S.O.), and by a Science Foundation Ireland Principal Investigator Award (11/PI/1066) (J.S.O.).
Authorship
Contribution: All authors drafted the first version of different sections of the manuscript and critically reviewed the final manuscript.
Conflict-of-interest disclosure: R.J.S.P. has received honoraria from Octapharma and has received grant awards from Novo Nordisk and Bayer. J.S.O. has served on the speaker’s bureau for Baxter, Bayer, Novo Nordisk, Boehringer Ingelheim, Leo Pharma, and Octapharma; has served on the advisory boards of Baxter, Bayer, Octapharma, CSL Behring, Daiichi Sankyo, Boehringer Ingelheim, and Pfizer; and has received research grant funding awards from Baxter, Bayer, Pfizer, and Novo Nordisk. The remaining authors declare no competing financial interests.
Correspondence: James S. O’Donnell, Haemostasis Research Group, Institute of Molecular Medicine, St. James’s Hospital, Trinity College Dublin, Dublin 8, Ireland; e-mail: jodonne@tcd.ie.
References
Author notes
J.M.O. and R.J.S.P. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal