Abstract
Fanconi anemia (FA) is the most frequent inherited cause of bone marrow failure (BMF). Most FA patients experience hematopoietic stem cell attrition and cytopenia during childhood, which along with intrinsic chromosomal instability, favor clonal evolution and the frequent emergence in their teens or young adulthood of myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML). To early identify and further predict bone marrow (BM) clonal progression and enable timely treatment, the follow-up of FA patients includes regular BM morphological and cytogenetic examinations. Allogeneic hematopoietic stem cell transplantation (HSCT) remains the only curative treatment of FA patients with MDS or AML. Although questions remain concerning HSCT itself (including the need for pretransplant chemotherapy, the best conditioning regimen, and the optimal long-term follow-up of such patients especially regarding secondary malignancies), clonal evolution in the absence of significant BM dysplasia and blast cells can be difficult to address in FA patients, for whom the concept of preemptive HSCT is discussed. Illustrated by 3 representative clinical vignettes showing specific features of MDS and AML in FA patients, this paper summarizes our practical approach from diagnosis through treatment in this particular situation.
Medscape Continuing Medical Education online
This activity has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education through the joint providership of Medscape, LLC and the American Society of Hematology.
Medscape, LLC is accredited by the ACCME to provide continuing medical education for physicians.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test with a 75% minimum passing score and complete the evaluation at http://www.medscape.org/journal/blood; and (4) view/print certificate. For CME questions, see page 3105.
Disclosures
Laurie Barclay, freelance writer and reviewer, Medscape, LLC, owns stock, stock options, or bonds from Pfizer. Editor Nancy Berliner and the authors declare no competing financial interests.
Learning objectives
Distinguish the clonal evolution and development of myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML) in patients with Fanconi anemia, based on a review.
Evaluate the diagnostic workup of and follow-up for development of MDS and AML in patients with Fanconi anemia.
Determine the management of MDS and AML in patients with Fanconi anemia.
Release date: June 16, 2016; Expiration date: June 16, 2017
Introduction
Fanconi anemia (FA) is the most frequent genetic cause of bone marrow failure (BMF).1 More than 18 FA genes have been identified, with FANCA, FANCC, FANCG, and FANCD2 being the most frequently involved in patients.2-4 The natural history of FA is marked by progressive marrow failure during early childhood, and the diagnosis is frequently done at this stage using the chromosome breakage test in blood.1 Throughout life, the hematopoietic situation may change spontaneously by genetic reversion with “somatic mosaicism”5 or by clonal evolution toward myelodysplastic syndrome (MDS) or acute myeloid leukemia (AML).6,7 Biologically, these MDS and AML are characterized by underrepresentation of the common cytogenetic and molecular subgroups and by overrepresentation of a specific pattern of unbalanced chromosomal translocations leading to copy-number abnormalities.6,7 The rate of solid cancers in adult FA patients is also considerably higher.8,9 A special form of the disease is seen in the rare children with biallelic BRCA2/FANCD1 and PALB2/FANCN mutations, who tend to develop MDS, AML, and/or solid cancers (particularly Wilms tumors and medulloblastomas) in very early life.10-13
Allogeneic hematopoietic stem cell transplantation (HSCT) remains the only curative treatment of MDS or AML in FA14-16 but must be carefully considered based on clinical and biological criteria and other decision components such as donor immune compatibility, procedure toxicity, and the higher risk of solid tumors after HSCT. In addition, the concept of preemptive transplant, to preclude the development of leukemia or MDS, has not been fully addressed in specific clonal evolution cases.
This paper examines 3 representative cases and shares our experiences by reviewing recent data on the biology and staging of clonal evolution in FA patients and their clinical course upon treatment with contemporary HSCT protocols.
Patient cases
Patient 1
A 5-year-old girl was diagnosed with FA through her family history (an older brother with FA), short stature, skin café-au-lait spots, and a positive chromosomal breakage test. Two heterozygous deleterious FANCA mutations were found. At diagnosis, complete blood cell counts (CBCs) and bone marrow (BM) aspiration with morphological and karyotype analyses were normal. Regular follow-up (FU), including marrow aspiration every 18 months, was initiated. Clonal evolution with partial duplication of chromosome 1q was detected on karyotype 5 years later (46,XX,dup(1)(q21q42)[20]), whereas BM morphology and CBCs remained normal. When diagnosed at aged 19 years with a cytomegalovirus primary infection, her CBCs showed a hemoglobin level of 10 g/dL, a neutrophil count of 0.5 × 109/L, and a platelet count of 88 × 109/L. BM was hypoplastic without dysmorphic features. Cytogenetic analyses showed an additional unbalanced translocation leading to a partial 3q duplication (46,XX,dup(1)(q21q42)[2]/46,sl,der(1)t(1;3)(p36;q21)[16]).
Patient 2
A 15-year-old girl presented with moderate aplastic anemia. CBCs showed a hemoglobin level of 10 g/dL, a neutrophil count of 1.2 × 109/L, and a platelet count of 40 × 109/L. Skin café-au-lait spots were seen on the abdomen. Blood chromosomal breakage test was positive. Marrow aspiration was hypoplastic without karyotype abnormality. She was treated with androgens (norethandrolone) until she was pregnant 12 years later. She required red blood cell and platelet transfusions during pregnancy and delivered a healthy baby boy. Six months later, CBCs returned to their steady state, showing a hemoglobin level of 10g/dL, a neutrophil count of 1.5 × 109/L, and a platelet count of 70 × 109/L. She was lost to FU until she again needed monthly red blood cell transfusions at age 43 years. BM was hypoplastic without dysmorphic features. Cytogenetic analyses showed clonal evolution on karyotype (46,XX,i(7)(q10)[9]/46,idem,del(11)(q14)[3]). A new biological workup was performed to reevaluate the underlying diagnosis of FA made more than 25 years earlier. FA was definitively confirmed using skin fibroblasts, based on mitomycin C hypersensitivity, an “FA core” pattern, and 2 deleterious FANCA mutations.1 7
Patient 3
A short 11-year-old girl with low CBCs was diagnosed with FA through a positive chromosomal breakage test. Two heterozygous deleterious FANCG mutations were found. Her marrow was hypoplastic without dysmorphic features. BM karyotype and fluorescence in situ hybridization (FISH) screening using 7q and 3q probes did not identify any clonal cells. She was receiving red blood cell transfusions every 6 weeks and was considered for HSCT, but no matched donor was available and little was known at the time about alternative transplantation, (ie, mismatched or cord blood [CB] HSCT). Four years later, her hematologic counts improved for no clear reason, because morphological and cytogenetic BM analysis remained normal and the chromosomal breakage test was still positive. Five years later, at age 21 years, pancytopenia was again found, with a hemoglobin level of 7 g/dL, a neutrophil count of 0.5 × 109/L, and a platelet count of 27 × 109/L. BM was hypercellular and exhibited 25% blast cell infiltration, leading to the diagnosis of AML. The karyotype formula was 46,XX,der(10)t(3;10)(q23;q26),der(13)t(1;13)(q10;p10)[20], with 1q and partial 3q gains confirmed by array comparative genomic hybridization (aCGH), as well as a short RUNX1 intragenic deletion that was undetectable by standard karyotype.
Clonal evolution, MDS, and AML in FA patients
Pathogenesis and presentation
Studies in FA patients and in mice models support the view that DNA damage and cell stress trigger senescence and cell death in proliferative hematopoietic progenitor cells, with compensatory or inflammatory-related mobilization and eventual attrition of hematopoietic stem cells.18-21 The resulting stem and progenitor cell deficiency, the FA-intrinsic genetic instability, and possibly chronic inflammation are thought to create strong pressure toward clonal evolution in the BM of FA patients.21-23
The incidence of MDS in FA is reported to be 11% to 34% in 3 separate cross-sectional studies.6,24,25 The cumulative incidence of AML ranges from 10% to 37% by age 50 years, with the most common time to develop leukemia being in the teenage years or young adulthood.6,8,9,26 Clinically, MDS and AML in FA are often, but not always, preceded by a BMF phase. MDS usually presents as refractory cytopenia with multilineage dysplasia (World Health Organization 2008 classification), with or without excess of blasts.7,27 In pediatric classification, BM disorders of inherited origin, including FA, are separated from primary (presumably nongenetic), hypocellular refractory cytopenia in childhood (by the European Working Group of MDS in Childhood and the provisional pediatric World Health Organization 2008 classifications).28,29 It is important to note that for therapeutic decision making, a certain level of dyserythropoiesis is almost constant in FA patients, and a mild dyserythropoiesis should not be considered an MDS criterion in this population.27 Although AML can be diagnosed de novo, more often it develops from an MDS phase with an increasing proportion of blast cells over months or years. Isolated cases of acute lymphoblastic leukemia (ALL) were exceptionally reported.30,31 Throughout MDS and AML evolution, a typical pattern of acquired, nonrandom karyotypic abnormalities is associated with BM clonal evolution.6,7,27,32-34 Although classical de novo translocations such as t(8;21), t(15;17), and MLL translocations are virtually absent, unbalanced translocations and partial chromosome arm duplications or deletions are most frequent, resulting in gains or losses of chromosomal regions.7,27,34 The most frequent abnormalities are the gain of chromosome 1q (referred to here as +1q), 3q26q29 (+3q), deletion 7q (−7q), and RUNX1 gene abnormalities at 21q22 (RUNX1-abn), followed less frequently by 5q, 13q, and 20q deletions, whereas mutations involving genes other than RUNX1 are infrequent.7,27,34
FA reversion and somatic mosaicism
A special situation seen in FA, known in the field as somatic mosaicism, should be distinguished from clonal evolution. Somatic mosaicism results from a spontaneous genetic reversion of 1 of the mutated FANC alleles.5,35 Because revertant cells are functionally corrected, they have a growth advantage and can expand as an alternative source of hematopoiesis. The search for somatic mosaicism has been performed mainly in peripheral blood cells using the chromosomal breakage test (ie, in T cells under phytohemagglutinin stimulation), but mosaicism restricted to myeloid lineages is at least theoretically possible. Clinically, somatic mosaicism in HSPCs can improve or stabilize blood cell counts, resulting in a “natural gene therapy,” and should not be treated per se.5,35-37 However, clonal evolution from nonrevertant bystander cells into MDS/AML remains possible,38 and somatic mosaicism, just like HSCT, does not offer protection from solid cancers. Importantly, somatic mosaicism can confound the various FA blood tests, and for this and other reasons, many FA laboratories (including ours) favor, when possible, analyses on primary skin fibroblasts, despite the greater workload this entails.5,17,36,39 Finally, genetic reversion has not been seen in MDS and AML cells in FA, suggesting that this mechanism is not a common step in tumor progression.7 This latter feature has therapeutic consequences, because MDS/AML cells in FA will still be sensitive to reduced doses of chemotherapy when compared with the high toxicity of conventional doses in this population.
Diagnostic workup and FU
BM monitoring of clonal evolution and tumor progression in FA
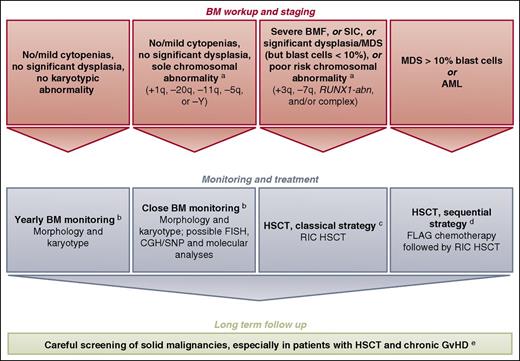
Blood cell counts and BM aspirates with morphological and conventional karyotype examinations should be performed regularly in FA patients to detect clonal progression (Figure 1; see legend for timing).40 In addition, a rapid change in blood cell counts at any time may reflect transformation and should lead to BM evaluation. BM dysplasia, especially in children, warrants careful evaluation by a hematopathologist with expertise in inherited BMF and MDS/AML predisposition syndromes.28,29,41 Regarding cytogenetic abnormalities, 1q gains and 7q deletions are easy to detect with a conventional BM karyotype, whereas 3q gains and RUNX1 abnormalities at 21q22 can be cryptically embedded in the karyotype and underdiagnosed.7,27,34 Additional FISH, aCGH/single-nucleotide polymorphism, and conventional or next-generation sequencing (NGS)-based techniques can be helpful in characterizing complex chromosome lesions and/or in revealing cryptic lesions like 3q and RUNX1 abnormalities.7,27,34 FISH and NGS may also help to detect minor clonal populations, the interest of which is to be established for the patient. If SNP analyses are performed, one must be aware that regions of homozygosity arising from consanguinity (ie, not somatic lesions) may be confounding in some patients, as has been demonstrated by paired fibroblast analysis.7
How we diagnose and manage FA patients with MDS and AML.aWe defined provisional cytogenetic/molecular categories by reference to MDS/AML literature in FA6,7,27,33,34 and non-FA49,50 patients (also see text). RUNX1-abn can include RUNX1 gene mutation, deletion, and/or translocation.7 Genetic reversion (hematopoietic somatic mosaicism) is not indicated in the figure to keep it simple. bThe timing of BM monitoring is discussed, especially because repeated aspiration is poorly tolerated in children, adolescents, and young adults.40 The overall consensus is that a 1-year basis of BM aspirate is reasonable and should be adapted in response to blood cell count changes, myelodysplasia signs, increased blast proportion, and/or cytogenetic evidence of clonal evolution. Conversely, BM monitoring is likely to be slightly delayed in FA children under age 10 years (except in BRCA2/FANCD1 patients), given the rarity at this age and relatively slow pace of clonal progression. cThe classical reduced-intensity conditioning (RIC) regimen consists of 90 mg/m2 of fludarabine (30 mg/m2 on days −4, −3, and −2) and 40 mg/kg of cyclophosphamide (10 mg/kg on days −5, −4, −3, and −2) in the case of matched related donor. One might argue that, in the case of MDS/AML, low-dose cyclophosphamide/fludarabine alone would not suffice as conditioning therapy due to the substantial number of residual host cells early after transplant with this approach and the risk of relapse; an alternative is the use of TBI (2-3 Gy) in patients with MDS/AML and an HLA- matched sibling donor.16,48 In the case of matched unrelated donor, the conditioning regimen consists of fludarabine (120 mg/m2), cyclophosphamide (40 mg/kg), and TBI (2 Gy). GVHD prophylaxis consists of mycophenolate acid and cyclosporine. Anti-thymocyte globulin is used in total doses of 5 mg/kg in the case of matched unrelated donor only. Others favor ex vivo T-cell depletion with an add-back of T cells to achieve a fixed graft T-cell dose of 1 × 105 CD3 cells per kilogram recipient.62 In the case of CB HSCT, we do not use anti-thymocyte globulin in the conditioning regimen. dOthers do not recommend cytoreduction, except in patients with BRCA2 mutations16,48,62 ; the sequential strategy comprising pretransplant chemotherapy with fludarabine (30 mg/m2 per day for 5 days) and cytarabine (1 g/m2 twice per day for 5 days) with granulocyte colony-stimulating factor injections (FLAG), followed 3 weeks later by an RIC regimen (4 days of cyclophosphamide, 10 mg/kg; 4 days of fludarabine, 30 mg/m2; and TBI, 2 Gy) delivered during chemotherapy-induced aplasia. Again, anti-thymocyte globulin is used in total doses of 5 mg/kg in the case of matched unrelated donor only. In the case of CB HSCT, we do not use anti-thymocyte globulin in the conditioning regimen. eScreening for malignancies, including oropharyngeal, dental, and gynecological examinations, forms part of long-term patient care. Long-term multidisciplinary surveillance is also mandatory for all patients post-HSCT.40 The multiple problems in early age, subsequent requirements for HSCT, and continuing poor prognosis in survivors due to cancer susceptibility are a source of stress for FA patients and their families. Adequate psychosocial support and a coordinated, multidisciplinary team with dedicated physicians are the cornerstones to successful management.40 CGH, comparative genomic hybridization; FLAG, fludarabine/cytarabine/granulocyte colony-stimulating factor; GVHD, graft-versus-host disease; SIC, severe isolated cytopenia; SNP, single nucleotide polymorphism.
How we diagnose and manage FA patients with MDS and AML.aWe defined provisional cytogenetic/molecular categories by reference to MDS/AML literature in FA6,7,27,33,34 and non-FA49,50 patients (also see text). RUNX1-abn can include RUNX1 gene mutation, deletion, and/or translocation.7 Genetic reversion (hematopoietic somatic mosaicism) is not indicated in the figure to keep it simple. bThe timing of BM monitoring is discussed, especially because repeated aspiration is poorly tolerated in children, adolescents, and young adults.40 The overall consensus is that a 1-year basis of BM aspirate is reasonable and should be adapted in response to blood cell count changes, myelodysplasia signs, increased blast proportion, and/or cytogenetic evidence of clonal evolution. Conversely, BM monitoring is likely to be slightly delayed in FA children under age 10 years (except in BRCA2/FANCD1 patients), given the rarity at this age and relatively slow pace of clonal progression. cThe classical reduced-intensity conditioning (RIC) regimen consists of 90 mg/m2 of fludarabine (30 mg/m2 on days −4, −3, and −2) and 40 mg/kg of cyclophosphamide (10 mg/kg on days −5, −4, −3, and −2) in the case of matched related donor. One might argue that, in the case of MDS/AML, low-dose cyclophosphamide/fludarabine alone would not suffice as conditioning therapy due to the substantial number of residual host cells early after transplant with this approach and the risk of relapse; an alternative is the use of TBI (2-3 Gy) in patients with MDS/AML and an HLA- matched sibling donor.16,48 In the case of matched unrelated donor, the conditioning regimen consists of fludarabine (120 mg/m2), cyclophosphamide (40 mg/kg), and TBI (2 Gy). GVHD prophylaxis consists of mycophenolate acid and cyclosporine. Anti-thymocyte globulin is used in total doses of 5 mg/kg in the case of matched unrelated donor only. Others favor ex vivo T-cell depletion with an add-back of T cells to achieve a fixed graft T-cell dose of 1 × 105 CD3 cells per kilogram recipient.62 In the case of CB HSCT, we do not use anti-thymocyte globulin in the conditioning regimen. dOthers do not recommend cytoreduction, except in patients with BRCA2 mutations16,48,62 ; the sequential strategy comprising pretransplant chemotherapy with fludarabine (30 mg/m2 per day for 5 days) and cytarabine (1 g/m2 twice per day for 5 days) with granulocyte colony-stimulating factor injections (FLAG), followed 3 weeks later by an RIC regimen (4 days of cyclophosphamide, 10 mg/kg; 4 days of fludarabine, 30 mg/m2; and TBI, 2 Gy) delivered during chemotherapy-induced aplasia. Again, anti-thymocyte globulin is used in total doses of 5 mg/kg in the case of matched unrelated donor only. In the case of CB HSCT, we do not use anti-thymocyte globulin in the conditioning regimen. eScreening for malignancies, including oropharyngeal, dental, and gynecological examinations, forms part of long-term patient care. Long-term multidisciplinary surveillance is also mandatory for all patients post-HSCT.40 The multiple problems in early age, subsequent requirements for HSCT, and continuing poor prognosis in survivors due to cancer susceptibility are a source of stress for FA patients and their families. Adequate psychosocial support and a coordinated, multidisciplinary team with dedicated physicians are the cornerstones to successful management.40 CGH, comparative genomic hybridization; FLAG, fludarabine/cytarabine/granulocyte colony-stimulating factor; GVHD, graft-versus-host disease; SIC, severe isolated cytopenia; SNP, single nucleotide polymorphism.
Diagnosis of an underlying (previously unknown) FA in patients with MDS or AML
An underlying FA diagnosis should be suspected in young patients with MDS or AML, especially when suggestive features are present, such as family history; physical abnormalities, including short size; spontaneous chromatid breaks; unbalanced 1q, 3q, or 7q translocations on BM karyotype; and/or excessive toxicity of usual chemotherapy. FA diagnosis may be technically difficult to confirm or exclude at the MDS/AML stage through blood tests, especially after chemotherapy. Analysis of the sensitivity to cross-linking agents of nonhematopoietic cells (ie, primary skin fibroblasts) is very helpful, but several weeks are required to grow the cells.17,39 Implementation of high-throughput NGS-based approaches42-44 should help to shorten the time needed to reveal FA in urgent situations like an overt AML presentation. Of note, patient mouth wash or saliva cells are highly contaminated by hematopoietic cells and are not a pure source of germ-line DNA.45 In summary, it remains the case that urgent therapeutic choices regarding chemotherapy doses need to be made based on clinical predictions of whether an underlying FA is more or less probable.
Outcome, current management, and treatment
Outcomes in FA patients with MDS or AML
Three single-center studies of more than 10 patients with FA and MDS/AML16,33,46 and 2 large multicenter studies14,15 have been published to date with a reasonable FU.
The adverse effect of morphological MDS and/or cytogenetic clonal abnormalities in FA patients was examined in a first retrospective study of 41 FA patients who had BM morphology and chromosomes reviewed by a single group in 2000.33 Patients were defined as “clonal” if they ever had a cytogenetic clone using a conventional marrow cell karyotype (FISH was not systematically performed in this study). The 5-year overall survival (OS) rate was 40% in those with abnormal cytogenetics vs 90% in those without a clone. Morphological MDS, independent of a cytogenetic clone, was found in 13 of 41 patients (32%) and was associated with a 5-year OS rate of 9%. No patients survived in the group when both morphological MDS and a cytogenetic clone where identified.33 Although both factors had an impact, especially when seen together, morphology was more potent than cytogenetics in predicting adverse outcomes in this study. In 2008, Ayas et al reported 11 patients with FA with MDS and/or clonal abnormalities, including 1 patient with clear AML (4 patients had only a cytogenetic clone), who were transplanted using cyclophosphamide and total body irradiation (TBI; 450 cGy).46 Ten patients were reported alive with no evidence of disease with a median FU of nearly 4 years. The overall excellent results illustrate the possibility of long-term survivors after HSCT in this situation. More recently, Mitchell et al reported 21 FA patients transplanted due to AML (n = 12), ALL (n = 1), and advanced MDS (n = 8).16 With a median FU of nearly 10 years, the 5-year OS rate was 33%, illustrating again that long-term remission can be achieved after HSCT in these patients, even in cases of overt leukemia.
Two large multicenter retrospective studies were published recently on patients with MDS/AML transplanted in the setting of FA. Data on 113 FA patients with cytogenetic abnormalities (n = 54), MDS (n = 45), or AML (n = 14) from 1985 to 2007 were analyzed by the Center for International Blood and Marrow Transplant Research.14 OS rates at 1, 3, and 5 years after transplant were 64%, 58%, and 55%, respectively. Younger patients and HLA-matched related-donor transplant recipients with cytogenetic abnormalities alone have better OS. The European Group for Blood and Marrow Transplantation analyzed 795 FA patients who underwent initial HSCT between 1972 and 2010, with 58 patients transplanted for MDS or AML.15 OS rates at 1, 3, and 5 years were 44%, 41%, and 35%, respectively. The cumulative incidence of relapse at 2 years was 11%. There was no difference in OS by donor type (related vs unrelated).
HSCT studies are summarized in Table 1. Overall, HSCT is the only definitive treatment of FA patients with MDS and AML, and currently offers a 30% to 40% long-term OS rate.47
Overview of studies that include more than 10 FA patients transplanted for MDS or AML
| Ref. . | Period . | Patients, N . | Disease . | Donor type . | Relapse . | Survival . |
|---|---|---|---|---|---|---|
| 46 | 2001-2007 | 11 | 4 Isolated cytogenetic abnormalities, 6 morphological MDS, and 1 AML | 9 MSD, 1 MMRD, and 1 CB | None | 10/11 patients; median FU 46 mo |
| 14 | 1985-2007 | 113 | 54 Isolated cytogenetic abnormalities, 45 morphological MDS, and 14 AML | 82 MSD, 16 URD, and 15 CB | N/A | 55% OS at 5 y; median FU 84 mo (survivors)* |
| 15 | 1972-2010 | 58 | N/A | 29 MSD, 2 other relative, and 27 URD | 11% CI of relapse | 35% OS at 5 y; median FU 49 mo |
| 16 | 1988-2011 | 21 | 8 Morphological MDS, 12 AML, and 1 ALL (6 BRCA2/D1 patients) | 2 MSD, 1 MMRD, 12 MUD, 4 CB, and 2 DCB | 24% CI of relapse (50% for BRCA2 patients) | 33% OS at 5 y; median FU 9.5 y |
| Ref. . | Period . | Patients, N . | Disease . | Donor type . | Relapse . | Survival . |
|---|---|---|---|---|---|---|
| 46 | 2001-2007 | 11 | 4 Isolated cytogenetic abnormalities, 6 morphological MDS, and 1 AML | 9 MSD, 1 MMRD, and 1 CB | None | 10/11 patients; median FU 46 mo |
| 14 | 1985-2007 | 113 | 54 Isolated cytogenetic abnormalities, 45 morphological MDS, and 14 AML | 82 MSD, 16 URD, and 15 CB | N/A | 55% OS at 5 y; median FU 84 mo (survivors)* |
| 15 | 1972-2010 | 58 | N/A | 29 MSD, 2 other relative, and 27 URD | 11% CI of relapse | 35% OS at 5 y; median FU 49 mo |
| 16 | 1988-2011 | 21 | 8 Morphological MDS, 12 AML, and 1 ALL (6 BRCA2/D1 patients) | 2 MSD, 1 MMRD, 12 MUD, 4 CB, and 2 DCB | 24% CI of relapse (50% for BRCA2 patients) | 33% OS at 5 y; median FU 9.5 y |
CI, cumulative incidence; DBC, double CB; MMRD, mismatched related donor; MSD, matched sibling donor; MUD, matched unrelated donor; Ref., reference; URD, unrelated donor.
Better results with matched sibling donor, younger age (≤14 y), and cytogenetic abnormality only.
HSCT indications in FA patients with clonal evolution
When a matched donor is available, the optimal time for HSCT outside clonal evolution is the need for transfusion support (severe BMF or severe isolated cytopenia).40,48 Patients with a level of genetic reversion (somatic mosaicism) but without severe aplasia or additional poor-prognosis clone do not need HSCT. Regarding FA patients with clonal evolution, decision-making criteria for HSCT include overt AML and MDS with excess of blast cells, significant dysplasia, and/or poor-prognosis cytogenetic abnormalities (Figure 1). Based on information in FA6,7,27,33,34 and non-FA49,50 patients, we have provisionally retained as poor-prognosis abnormalities −7q, +3q, complex karyotype, and RUNX1-abn. Conversely, +1q is a common and early somatic chromosomal abnormality in FA patients’ BM cells but can be seen without dysplasia and, by itself, does not seem to be associated with rapid transformation.6,7,27,33 Other abnormalities such as −5q, −11q, and −20q can be found, although more rarely, like in non-FA MDS patients in whom they are not considered poor-prognosis abnormalities.49,50 In such cases of an isolated chromosomal abnormality but absence of severe dysplasia or cytopenia, and depending on the patient context, one can consider postponing HSCT, but close FU is mandatory (Figure 1). In any case, a pre-HSCT workup that includes the search for a donor should not be delayed, and this information can eventually impact the therapeutic decision. Although evidenced-based medicine for prognosis is lacking, morphological and cytogenetic/molecular criteria will have to be carefully evaluated in long-term FU patient cohorts (not restricted to HSCT centers).
HSCT per se in FA patients with MDS and AML
Other than pre-HSCT cytoreduction, discussed in the next section, transplant itself should proceed the same way as for FA patients without clonal evolution (Figure 1). Historically, Gluckman and colleagues at Hôpital Saint-Louis identified an excessive regimen-related toxicity when HSCT was performed with the same conditioning regimen as for other BMF.51-53 Adapted conditioning regimens with low-dose cyclophosphamide with or without low-dose radiotherapy have been proposed since then.54,55 In recipients of HLA-matched family-donor HSCTs, excellent outcomes have been reported using a low-dose cyclophosphamide-based regimen alone56 or in association with fludarabine.57 Some families might consider having a healthy and HLA-compatible baby after selecting an embryo using preimplant genetic diagnosis.58,59 In the case of unrelated-donor HSCT, drastic improvements have been reported since 2000 due to better HLA typing and the use of fludarabine-based reduced-intensity conditioning regimens with or without T-cell depletion.15,60,61 HLA-matched unrelated donors are the preferred source of stem cells when no matched family donor exists. MacMillan et al recently reported 130 FA patients who underwent unrelated-donor HSCTs between 1995 and 2012.62 Patients without a history of opportunistic infection or transfusions before HSCT and who received conditioning with TBI (300 cGy), cyclophosphamide, fludarabine, and anti-thymocyte globulin have a 5-year survival probability of 94%. Of note, 37 patients (28%) were transplanted due to clonal abnormality before HSCT, and 10 patients (8%) were transplanted only because of late MDS/AML. Importantly, 31 patients (24%) received a CB HSCT in that study, and the same group recently reported 4 single CB transplants and 2 double CB-HSCTs in a total of 21 patients transplanted for overt AML or MDS.16 Alternative donors (ie, mismatched, CB, or more recently haplo-HSCT) may thus be considered, given the poor prognosis of such patients, but should be decided in accordance with the experience of each team.
Regarding the stem cell source, BM cells have been shown to be superior to peripheral blood stem cells (PBSCs) in patients with acquired aplastic anemia undergoing matched sibling or unrelated transplants,63-65 due to the higher risk of extensive chronic GVHD using PBSCs compared with BM. Specifically in FA patients, clonal evolution as an indication for HSCT, PBSCs as the stem cell source, and previous chronic GVHD (time-dependent) were all found to be independently associated with a higher risk of secondary malignancies and highly associated with secondary death.15 These results are sufficiently important to consider marrow the recommended source of stem cells in this particular population, even in cases of clonal evolution. Regarding GVHD prophylaxis, the most commonly used combination is cyclosporine plus mycophenolate mofetil.57,62,66
The role of pretransplant cytoreduction
FA patients who develop MDS and/or leukemia are not easy to manage because sensitivity to DNA-damaging agents limits the therapy they can tolerate.47 Chemotherapy in such patients is regularly associated with significant toxicity and a possible prolonged period of aplasia, with certain complications that may eventually contraindicate HSCT. One possibility is the use of sequential chemotherapy, which has been described in non-FA patients not in remission and has achieved acceptable results.67 The Cincinnati group first reported on the outcomes of 3 FA patients with AML and 1 with MDS/chronic myelomonocytic leukemia who received a reduced-intensity mini-FLAG regimen prior to HSCT.68 Only 1 patient survived AML-free, but FU only lasted 8 months (1 death through relapse and 2 from infections). We also reported our experience at Hôpital Saint-Louis using a sequential strategy in 6 FA patients (5 AML and 1 MDS) comprising pretransplant FLAG chemotherapy, followed 3 weeks later by reduced-intensity conditioning. At the time of publication (28 months of FU), all patients were alive and disease-free.69 Since then, 2 patients have died (1 relapse and 1 severe chronic GVHD). Two other AML patients received identical treatment: 1 died of infections in the first 100 days post-HSCT, whereas the other is doing perfectly well more than 2 years later (R.P.d.L., unpublished data). The Minnesota group recently reported their experience in 21 FA patients (12 AML, 1 ALL, and 8 advanced MDS), using low-dose cyclophosphamide with either busulfan or single-dose TBI, depending on transplantation era (1998-2011).16 Chemotherapy was used in 8 patients before HSCT, with only 3 remissions. Chemotherapy-associated toxicity separate from HSCT was seen in a total of 6 patients (mainly infections and persistent aplasia). Of the 8 patients who received pre-HSCT chemotherapy, only 2 were alive at time of reporting.
Overall, the role of pre-HSCT chemotherapy remains unclear in FA patients with MDS/AML. Published studies report on small numbers of highly heterogeneous patients with a variety of different therapies. Some subsets might benefit from cytoreduction before HSCT (ie, MDS with excess blasts/AML or BRCA2/FANCD1 patients). What remains clear is the risk of prolonged chemotherapy-associated aplasia, highlighting the importance of securing a donor before offering chemotherapy in these patients.
Long-term FU post-HSCT: new challenges
FA patients are at increased risk of malignancy post-HSCT (28% cumulative incidence of solid malignancy by age 50 years in the German Fanconi Anemia Registry).26 There is a 4.4-fold higher rate of squamous cell carcinomas in FA patients undergoing HSCT compared with FA patients of the same age who have not received an HSCT.70 Amid competing causes of mortality, the cumulative incidence of squamous cell carcinomas is between 24% at 15 years70 and 34% at 20 years.15 Although FA patients are inherently prone to develop tumors, chronic GVHD is also a key factor in their development post-HSCT.15,71 Clonal evolution was also identified as an independent risk factor for secondary malignancies, along with age older than 10 years at the time of HSCT, PBSCs as the stem cell source, and previous chronic GVHD, when considered as a time-dependent covariable.15 Thus, FA patients transplanted in cases of MDS or AML are at a particularly high risk of secondary cancers that are highly associated with mortality, and regular screenings for malignancies should therefore form part of long-term patient care (Figure 1).40 Such patients are systematically seen every 6 months at our clinic by gynecologists and stomatologists to enable early cancer detection and prompt surgery.72 Special attention to oral hygiene and personal habits such as limited alcohol consumption and smoking is mandatory. Although less documented, patients with FA are exposed to endocrine dysfunction after HSCT and warrant a comprehensive FU by a pediatric endocrinologist (considering hypothyroidism, diabetes and metabolic syndrome, and growth hormone deficiency, for example).73,74 Regarding fertility, viable pregnancy is rare but possible in FA patients before HSCT, as in patient 2, but also sometimes after transplant.75
Back to our patients: staging, treatment, and FU
Patient 1
This case illustrates a progressive clonal evolution in the BM, as shown by the serial acquisition of chromosomal translocations resulting in partial 1q duplication and partial 3q duplication 9 years later, both confirmed by FISH and aCGH. A sole chromosome 1q translocation or duplication can be seen in the early stages of BM clonal evolution in FA, including “normal” or hypoplastic BM, indicating that they are not associated per se with transformation.6,7 In our patient, the detection of +3q, known to be associated with later stages of BM progression and unfavorable prognosis,7,34 prompted us to proceed quickly (4 months later) to a matched unrelated HSCT donor. BM was still hypoplastic with only minor dysmorphic signs without excessive blasts, the reason why we did not proceed to cytoreduction pre-HSCT (Figure 1). In 2010, she received allele-matched (10/10) unrelated BM as the stem cell source after a fludarabine-based reduced-intensity conditioning regimen including 2-Gy TBI. We used in vivo T-cell depletion using anti-thymocyte globulin, although others favor ex vivo T-cell depletion with an add-back of T cells to achieve a fixed graft T-cell dose of 1 × 105 CD3 cells per kilogram recipient.62 The posttransplant period was associated with long-lasting immune deficiency, mainly complicated by multiple cytomegalovirus reactivations and a pulmonary aspergillosis, which eventually improved 2 years post-HSCT. The patient is still disease-free 4 years post-HSCT; however, she was recently diagnosed with a microinvasive squamous cell carcinoma of the uterine cervix. The systematic FU post-HSCT by a dedicated gynecologist enabled an early diagnosis of the cancer, which was cured by surgery. The potential detrimental effect of TBI regarding secondary cancer in FA remains a subject for discussion. Recent published data,62 as well as our personal experience (R.P.d.L., unpublished observations), favor using low-dose TBI and unrelated HSCTs in FA patients because of the high risk of rejection. Moreover, in the large European Group for Blood and Marrow Transplantation HSCT experience, TBI per se was not identified as an independent risk factor for secondary malignancies.15
Patient 2
This patient experienced the emergence of BM karyotype abnormalities at age 43 years, which highlights the persistent risk of BM progression at any age and the consequent need for regular lifelong evaluation to detect and treat clonal evolution in a timely manner. The BM morphology was subnormal, but the presence of chromosomal abnormalities on 7q and 11q and the need for transfusion led us to proceed with transplant with an allele-matched (10/10) unrelated donor in 2005, using BM as the stem cell source. The reduced-intensity conditioning regimen used at that time for FA patients and unrelated donor consisted of fludarabine, busulfan, cyclophosphamide, and anti-thymocyte globulin (without TBI). The patient engrafted promptly, and the early period post-HSCT was free of complications. At day 100, she was still being transfused with red blood cells due to erythroblastopenia arising from a major ABO donor–recipient incompatibility. Antidonor immunoglobulin M hemagglutinins persisted despite multiple treatments, including immunosuppressive therapy and plasmapheresis. Fifteen years later, at age 53 years, she has returned to work and a normal life, except for monthly red blood cell transfusions due to ABO immunization (full donor chimerism) and regular iron chelation therapy.
Patient 3
This patient represents an unusual, but not exceptional, case of a de novo AML at age 21 years. Previous BM monitoring by morphology and karyotype (the last BM assessment being 2 years before the AML diagnosis) did not detect any clonal evolution, whereas +1q, +3q, and RUNX1-abn were detected in AML cells. Only CB unit was available as a stem cell source (5 of 6 HLA-matched with the patient). Due to the AML status, we proceeded to a sequential strategy, comprising pretransplant chemotherapy (FLAG), followed 3 weeks later by a reduced-intensity conditioning regimen (Figure 1). This patient was the first to receive this original approach in our center in 2006.69 She tolerated the sequential regimen well and was engrafted promptly at day 21 for neutrophils and at day 29 for platelets, with a complete donor chimerism at day 100. She developed grade II skin GVHD, which responded well to 1 mg/kg of steroids. The patient remains in remission 10 years after HSCT, disease-free, and without any other complication arising from transplant.
Final considerations
FA is a chronic condition, with a complex and highly variable natural history from one patient to another.40,76 When clonal evolution is apparent in the BM, patients deserve a personalized therapeutic plan encompassing personal and disease-specific criteria. Although certain situations are indications for prompt transplant (overt AML, significant MDS, or cytogenetic/molecular abnormality of chromosome 3q, 7q, RUNX1, and/or complex), it remains unclear whether other patients with a level of isolated clonal evolution, such as a sole +1q clone, should receive immediate treatment (Figure 1). This situation and FA genetic reversion (called somatic mosaicism in the FA field) raise somewhat similar questions as the recently recognized clonal hematopoiesis of indeterminate potential in aging individuals, idiopathic cytopenia of unknown significance, and clonal cytopenia of unknown significance of the general (non-FA) population.77-79 The same limitations have also emerged in the criteria currently adopted to establish the diagnosis and to classify MDS.79 We therefore need longer FU on large cohorts of well-characterized FA patients not restricted to cohorts from HSCT centers, to extensively integrate the morphological, cytogenetic, and molecular data with survival prognoses. This information should help to better define the optimal timing for HSCTs or other options. In any case, this type of analysis faces a major limitation (and bias), because many teams proceed to HSCT upfront in cases involving abnormal karyotype, thus impacting directly on natural history.
As far as cytoreduction pre-HSCT is concerned, we all agree that toxicity is high in FA patients. However, certain specific subsets may benefit from pre-HSCT cytoreduction (ie, MDS with excess of blasts, overt AML, or BRCA2/FANCD1 patients). What remains clear is the risk of prolonged chemotherapy-associated aplasia, highlighting the importance of securing a donor before offering chemotherapy to these patients. Regarding HSCT itself, considerable progress has been made in the last 20 years (eg, matched unrelated donor selection, fludarabine-based conditioning regimen, and supportive care). A major goal of the procedure is to avoid chronic GVHD, which is detrimental for FA patients, highlighting the outstanding importance of using BM as source of stem cells in this setting, even in cases of clonal evolution. Lastly, alternative donors (ie, mismatched, CB, or haplo-HSCT) may be considered, given the poor prognosis of such patients, according to the experience of each center. In any case, FA patients should be carefully and regularly followed on a very long-term basis to enable early detection and prompt treatment of secondary cancers, which remain the major threat and complication in this particular population.
Acknowledgments
The authors thank Gérard Socié for helpful discussion and critical reading of the manuscript; colleagues from Hôpital Saint-Louis, Hôpital Robert Debré, and abroad for helpful discussion; and the Association Française pour la Maladie de Fanconi for their constant and helpful support.
Authorship
Contribution: R.P.d.L. and J.S. wrote and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Régis Peffault de Latour, Service d’Hématologie-Greffe, Hôpital Saint-Louis, 1 Ave Claude-Vellefaux, 75010 Paris, France; e-mail: regis.peffaultdelatour@aphp.fr; and Jean Soulier, Service d’Hématologie Biologique, Hôpital Saint-Louis, 1 Ave Claude-Vellefaux, 75010 Paris, France; e-mail: jean.soulier@sls.aphp.fr.