Key Points
Translocations between PD-L1 and the IGH locus represent a genetic mechanism of PD-L1 overexpression in DLBCL.
Genetic alterations in the PD-L1/PDL-2 locus are mainly associated with the non-GCB subtype of DLBCL.
Abstract
Diffuse large B-cell lymphoma (DLBCL) is one of the most common and aggressive types of B-cell lymphoma. Deregulation of proto-oncogene expression after a translocation, most notably to the immunoglobulin heavy-chain locus (IGH), is one of the hallmarks of DLBCL. Using whole-genome sequencing analysis, we have identified the PD-L1/PD-L2 locus as a recurrent translocation partner for IGH in DLBCL. PIM1 and TP63 were also identified as novel translocation partners for PD-L1/PD-L2. Fluorescence in situ hybridization was furthermore used to rapidly screen an expanded DLBCL cohort. Collectively, a subset of samples was found to be affected by gains (12%), amplifications (3%), and translocations (4%) of the PD-L1/PD-L2 locus. RNA sequencing data coupled with immunohistochemistry revealed that these cytogenetic alterations correlated with increased expression of PD-L1 but not of PD-L2. Moreover, cytogenetic alterations affecting the PD-L1/PD-L2 locus were more frequently observed in the non–germinal center B cell–like (non-GCB) subtype of DLBCL. These findings demonstrate the genetic basis of PD-L1 overexpression in DLBCL and suggest that treatments targeting the PD-1–PD-L1/PD-L2 axis might benefit DLBCL patients, especially those belonging to the more aggressive non-GCB subtype.
Introduction
Diffuse large B-cell lymphoma (DLBCL) is one of the most common and aggressive forms of B-cell lymphomas.1 At least 2 subtypes of DLBCL can be distinguished by gene expression analysis: the germinal center B cell–like (GCB) and the activated B cell–like (ABC) subtypes, with the latter being characterized by increased disease aggressiveness and worst clinical outcomes.2,3 In recent years, the incorporation of anti-CD20 therapeutic antibodies into the standard cyclophosphamide, doxorubicin, vincristine, prednisolone (CHOP) treatment has contributed to an overall improvement of patient survival.3-5 Despite this, disease relapse is often refractory to the currently available therapies, which marks the necessity for the development of new therapeutic approaches for DLBCL.6
Recently, the genomic characterization of DLBCL has been performed in several studies using next-generation sequencing (NGS) technologies. These and earlier studies contributed to the identification of a set of genes/pathways that are frequently targeted by mutations in DLBCL.7-12 Moreover, whole-genome sequencing (WGS) and RNA sequencing (RNAseq) enabled the identification of novel structural variations (SVs) in DLBCL involving the TP63 and CIITA genes.13-15 These translocations may contribute to tumorigenesis in addition to the few well-described, recurrent SVs such as rearrangements involving the proto-oncogenes BCL6, BCL2, and MYC and the immunoglobulin heavy-chain (IGH) gene locus.16-18
Translocations between IGH and proto-oncogenes usually result in upregulation of the latter by bringing them under the control of the potent IGH Eμ and 3′ enhancers.19 These events, especially in germinal cell (GC)-related B-cell lymphomas, have been suggested to be mainly triggered by aberrant, off-target activity of activation-induced cytidine deaminase (AID).20,21 The physiologic role of AID is to deaminate cytidine residues within the variable (V) or switch (S) regions of the IGH locus, which is essential for the somatic hypermutation (SHM) or class switch recombination (CSR) processes, respectively.22,23 During CSR, AID-induced mismatches lead to double-strand breaks (DSBs) in the S regions that are resolved by one of the 2 major DSB repair mechanisms, the nonhomologous end-joining (NHEJ) pathway.24 We have previously suggested that defects in the NHEJ pathway might be associated with the formation of translocations involving the IGH locus.25
PD-L1 and PD-L2 are signaling molecules expressed on the surface of antigen-presenting cells.26 Upon interaction with their receptor PD-1 on effector T cells, they transmit a negative regulatory signal that leads to functional anergy of the T cells.27 Various malignancies have been shown to overexpress PD-L1 to escape T cell–mediated killing.28-30
In recent years, the introduction of drugs targeting the PD-1–PD-L1/PD-L2 immune-modulatory pathway has shown promising results in the treatment of aggressive malignancies such as melanoma, renal cancer, and lung cancer.31 A study on Hodgkin lymphoma demonstrated that as much as 87% of patients with refractory disease responded to PD-1 blockade.32 Accordingly, the chromosome 9p24.1 cytoband, which includes the adjacent PD-L1 and PD-L2 genes, was found to be rearranged to the V regions of the IGH locus in an HL-derived cell line.33 In non-Hodgkin lymphomas, alterations affecting the 9p24.1 cytoband were observed in a subset of DLBCLs,33 but more frequently, in several specific types of large B-cell lymphomas, including primary mediastinal large B-cell lymphomas (PMBCL), primary testicular lymphoma (PTL), and primary nervous system lymphoma (PNSL).33-35 PD-L1 overexpression was furthermore observed in the aggressive ABC/non-GCB subtype of DLBCL.36,37 Moreover, a recent study performed on DLBCL-derived cell lines demonstrated that PD-1 blockade restores T-cell function in vitro.38 Together, the PD-1–PD-L1/PD-L2 axis may constitute a target for immunotherapy in refractory and aggressive DLBCL.
To investigate the incidence of structural variants affecting the PD-L1/PD-L2 locus in DLBCL and to further understand the genetic mechanisms of PD-L1 deregulation in these tumors, we performed WGS, RNAseq, and cytogenetic analysis in samples from several cohorts of patients with DLBCL. Copy number gains, amplifications, and translocations targeting the PD-L1/ PD-L2 locus were observed in a subset of tumor samples, which resulted in overexpression of the PD-L1 gene.
Methods
Patient cohorts
Chinese cohort.
Frozen material corresponding to tumor biopsies from 176 Chinese patients with DLBCL were obtained from the Sun Yat-Sen University Cancer Center and the Tianjin Medical University Cancer Institute and Hospital. Matching peripheral blood DNA samples were available for 76 patients. The Epstein-Barr virus (EBV) active infection status was assessed in a subset of samples using chromogenic in situ hybridization. In addition, a tissue microarray with 100 Chinese DLBCLs was purchased from US Biomax Inc (Rockville, MD).
Swedish cohort.
Samples from 64 Swedish patients with DLBCL were collected at the Uppsala University Hospital. Formalin-fixed paraffin-embedded (FFPE) tissue was available and tumor tissue cores were organized in tissue microarrays. DNA and RNA from 3 samples were extracted from the FFPE blocks.
American cohort.
FFPE tissue sections were available from 26 DLBCL samples from the database of the Herbert Irving Comprehensive Cancer Center. Frozen tissues from 2 tumors were used for the extraction of DNA and RNA.
These 3 cohorts of samples were reviewed by experienced pathologists in their respective centers. PMBCLs were excluded based on site of involvement, morphology, and, in some cases, an additional immunostaining against CD30. Samples were further classified as GCB and non-GCB by immunohistochemistry (IHC) according to the Hans algorithm.39 For a subset of American samples, GCB and ABC classification based on gene expression analysis was also available.40 A summary of the experiments performed, results, and available data are provided in supplemental Figure 1 (available on the Blood Web site). The institutional review board at the Karolinska Institutet approved this study.
DNA extraction, WGS, and analysis of SV
DNA was isolated either with the DNeasy Tissue and Blood Kit (QIAGEN, Venlo, The Netherlands) or the Recoverall Total Nucleic Acid Isolation kit (Ambion-Life Technologies, Carlsbad, CA). WGS was performed using either the Illumina HiSequation 2000 or Hiseq X10 platform (Illumina, San Diego, CA).9,41,42 SeekSV, an in-house method, was used for the detection of SVs. Further details are provided in the supplemental Methods.
RNA extraction, RNAseq, and quantitative real-time polymerase chain reaction
Total RNA was extracted either with Trizol (Life Technologies), the RNeasy kit (QIAGEN) or the Recoverall Total Nucleic Acid Isolation kit. Library preparation and the RNAseq method on the Illumina HiSequation 2000 platform have been described before.43 The number of fragments per kilobase of transcript per megabase of mapped reads (FPKM) was used to determine relative gene expression levels. Detection of fusion transcripts was enabled by SOAPfuse.44 RNAseq data were also used for the classification of the 2 disease subtypes, GCB and ABC, based on a published set of genes.45 Information about qPCR is provided in the supplemental Methods.
Detection of cytogenetic alterations by fluorescence in-situ hybridization
Fluorescent probes flanking the PD-L1/PD-L2 locus were generated from bacterial artificial chromosome clones RP11-963L, RP11-12D24, RP11-207C16, and RP11-845C2, and labeled with spectrum green and orange dUTPs (Enzo Life Sciences, Farmingdale, NY). Vysis LSI IGH (Abbott Molecular, Abbott Park, IL) break-apart rearrangement probes were used for analysis of the IGH locus. Treatment and probe hybridization were performed as previously described.46 Further details are provided in the supplemental Methods.
Detection of PD-L1, human leukocyte antigen class I, and CD8 by IHC
The antibodies used for IHC were a PD-L1 monoclonal antibody (#13684, clone E1L3N; Cell Signaling, Beverly, MA), a PD-L2 polyclonal antibody (SAB3500395; Sigma-Aldrich, St. Louis, MO), a CD8 monoclonal antibody (M7103, clone C8/144B; DAKO), and the human leukocyte antigen (HLA) I assessing antibodies HCA2 and HC1047-49 (Nordic MUbio, Susteren, The Netherlands). Further details are provided in the Supplemental Materials and methods.
Results
WGS reveals rearrangements involving the PD-L1/PD-L2 locus
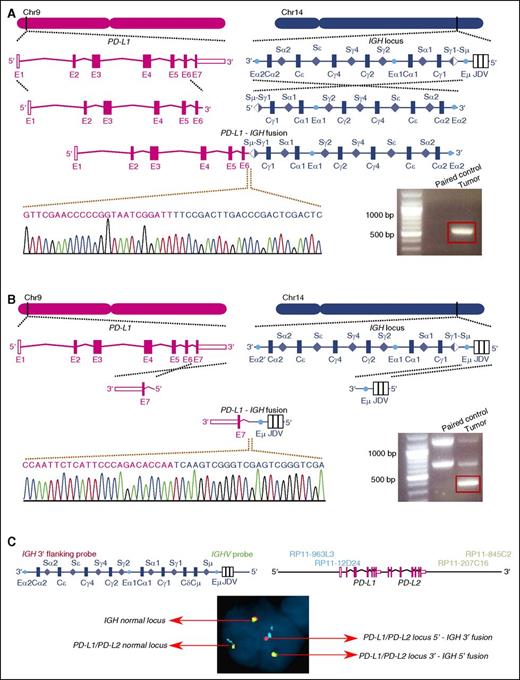
WGS was performed on DNA from 20 Chinese DLBCL biopsy samples and their respective paired normal samples in 2 sets of experiments (supplemental Figure 2A-B). The mean sequencing depths were 39.5× and 35.1× for the first and second set, respectively. The percentage of bases covered by at least 10 reads was 97.7% for the first and 97.9% for the second set. Structural rearrangement analysis demonstrated the presence of balanced, somatically acquired translocations between the IGH and the PD-L1 loci in one of the samples, DL48. This is, to our knowledge, the first time that PD-L1 is identified as a fusion partner for IGH in DLBCL. The finding of WGS was subsequently validated by breakpoint-specific polymerase chain reaction (PCR) analysis using primers surrounding the fusion points between the 2 loci (Figure 1A-B). The breakpoint within the IGH locus was situated in the Sμ region, which was already rearranged to the Sγ1 region through CSR, whereas on chromosome 9, the breakpoint was located in the intron separating the sixth and seventh exons of the main transcript of PD-L1 (NM_014143). Of note, AID-targeting motifs (WRC/GYW) were furthermore identified on both sides of the breakpoints, suggesting that illicit activities of AID during CSR might be responsible for the initiation of this translocation event (supplemental Figure 3). Fluorescence in situ hybridization (FISH) analysis on FFPE slides derived from the DL48 tumor showed the presence of split signals of PD-L1 and IGH loci in 52% and 46% of the nuclei counted, respectively. This supports the presence of the rearrangement in the main tumor clone. An additional FISH experiment using 4-color probes further demonstrated the fusion between the 2 loci and the balanced nature of the rearrangements (Figure 1C).
Identification of the PD-L1–IGH translocation in sample DL48. (A-B) The positions of the breakpoints in the 2 loci are indicated. The translocation is balanced, resulting in 2 distinct fusion chromosomes. Breakpoint-specific PCR and Sanger sequencing confirm the WGS results. The red box indicates the PCR product with expected size. The 100-bp plus ladder from Life Technologies was used. (C) FISH analysis of sample DL48 shows the colocalization of the probe signals of PD-L1 and IGH. The 5′ end of IGH appears in green, and the 3′ end in red. The probe targeting the 5′ end of the PD-L1/PD-L2 locus was labeled in cyan and the probe targeting the 3′ end in gold.
Identification of the PD-L1–IGH translocation in sample DL48. (A-B) The positions of the breakpoints in the 2 loci are indicated. The translocation is balanced, resulting in 2 distinct fusion chromosomes. Breakpoint-specific PCR and Sanger sequencing confirm the WGS results. The red box indicates the PCR product with expected size. The 100-bp plus ladder from Life Technologies was used. (C) FISH analysis of sample DL48 shows the colocalization of the probe signals of PD-L1 and IGH. The 5′ end of IGH appears in green, and the 3′ end in red. The probe targeting the 5′ end of the PD-L1/PD-L2 locus was labeled in cyan and the probe targeting the 3′ end in gold.
The fusion transcript that resulted from the translocation of the PD-L1 and IGH loci was subsequently identified by RNAseq and confirmed by PCR and Sanger sequencing. This transcript contained the first 6 exons of PD-L1 together with the third exon of IGHG1. The last exon of PD-L1, which was missing from this fusion transcript, encodes for the final 7 amino acids of the peptide chain, located in the intracellular domain,50,51 without any known function.27 RNAseq reads for each PD-L1 exon (supplemental Figure 4A) and an allele-specific quantitative PCR (qPCR) approach further illustrated that the majority of PD-L1 expression in this tumor originated from the fusion transcript, suggesting that the translocation is a driver event for PD-L1 expression (supplemental Figure 4B).
The WGS analysis of the 20 Chinese DLBCLs furthermore discovered a second translocation juxtaposing the PD-L1/PD-L2 locus to TP63. This translocation was also balanced and the breakpoints were located between the fifth and sixth exons of PD-L1, and the first and second exons of TP63 (supplemental Figure 5A-B). Moreover, a number of copy number alterations were discovered and are summarized in supplemental Figure 6.
Cytogenetic analysis on extended DLBCL cohorts
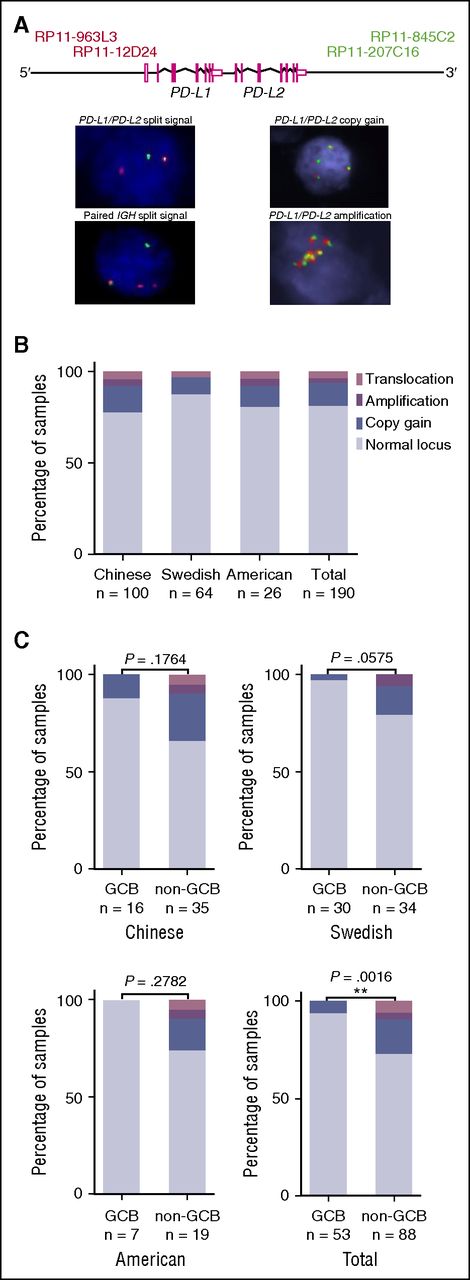
We used a FISH assay to identify additional cytogenetic alterations affecting the 9p24.1 cytoband, where the PD-L1 and PD-L2 genes are located adjacent to each other. A total of 179 samples, derived from 3 cohorts of patients (supplemental Figure 1), were analyzed. Of those, 23 cases (13%) presented with copy number gains in the PD-L1/PD-L2 locus, 3 cases (2%) with amplifications and 6 cases (3%) with split signals indicative of translocations. Among the latter 6 samples, split signals were also observed at the IGH locus in 3 samples (including DL48). Representative examples of cytogenetic alterations identified by FISH are presented in Figure 2A. The collective results from the 190 samples of which the status of the PD-L1/PD-L2 locus was analyzed by FISH and/or WGS identified 23 gains (12%), 5 amplifications (3%), and 7 translocations (4%) (Figure 2B). In samples that underwent both FISH and WGS, the data concerning the status of the PD-L1/PD-L2 locus were concordant (supplemental Figure 6).
Screening for cytogenetic alterations in the PD-L1/PD-L2 locus by FISH and WGS. FISH was performed on 179 samples and WGS on 24 samples. A number of samples underwent both analyses. In total, the status of the PD-L1/PD-L2 locus was made known across 190 DLBCL samples. (A) FISH probes and examples of cytogenetic alterations. The probes targeting the 5′ end of the PD-L1/PD-L2 locus were labeled in red and those targeting the 3′ end in green. A split signal indicative of a translocation is characterized by the lack of colocalization of the green and red probes within a nucleus. Gain is defined as the presence of 3 to 4 target loci within a cell, whereas amplification corresponds to ≥5 copies of the loci within a cell. (B) Distribution of PD-L1/PD-L2 translocations, gains, and amplifications across the different cohorts investigated. Data acquired by FISH and WGS. (C) Distribution of alterations in the PD-L1/PD-L2 locus in the 2 disease subtypes across the different cohorts. Fisher exact test was used for comparison of the frequency of these alterations between GCB and non-GCB samples.
Screening for cytogenetic alterations in the PD-L1/PD-L2 locus by FISH and WGS. FISH was performed on 179 samples and WGS on 24 samples. A number of samples underwent both analyses. In total, the status of the PD-L1/PD-L2 locus was made known across 190 DLBCL samples. (A) FISH probes and examples of cytogenetic alterations. The probes targeting the 5′ end of the PD-L1/PD-L2 locus were labeled in red and those targeting the 3′ end in green. A split signal indicative of a translocation is characterized by the lack of colocalization of the green and red probes within a nucleus. Gain is defined as the presence of 3 to 4 target loci within a cell, whereas amplification corresponds to ≥5 copies of the loci within a cell. (B) Distribution of PD-L1/PD-L2 translocations, gains, and amplifications across the different cohorts investigated. Data acquired by FISH and WGS. (C) Distribution of alterations in the PD-L1/PD-L2 locus in the 2 disease subtypes across the different cohorts. Fisher exact test was used for comparison of the frequency of these alterations between GCB and non-GCB samples.
Information about the molecular subtype was available for 141 of the samples analyzed by FISH and/or WGS. Results from all cohorts showed that cytogenetic alterations affecting the PD-L1/PD-L2 locus, including copy number gains, amplifications, and translocations, are more frequent in the non-GCB subtype (n = 24/88, 27% vs 3/53, 6% in GCB-type cases; Fisher exact test, P = .0016; Figure 2C). Notably, translocations and amplifications in the PD-L1/PD-L2 locus were exclusively found in non-GCB samples (Figure 2C) or ABC samples in the American cohort, in which GCB/ABC classification based on gene expression array was available.
WGS of selected samples in the expanded cohort
To further characterize the additional samples presenting a break or amplification of the PD-L1/PD-L2 locus identified by the FISH analysis, WGS was performed on tumor samples from 4 of the 7 affected patients, for which DNA was available (SL24, SL55, 2168, and 2171). A mean sequencing depth of 32.2× was achieved and 96.4% of the genome was covered by at least 10 reads (supplemental Figure 2C). WGS and breakpoint-specific PCR confirmed the existence of translocations in the PD-L1/PD-L2 locus in two of these samples (2168 and SL55) and identified the translocation partners. Sample 2168 had a breakpoint in the intergenic region between PD-L1 and PD-L2. Interestingly, the translocation partner here was PIM1, a proto-oncogene that is recurrently mutated in DLBCL and constitutes a target of aberrant SHM52 (supplemental Figure 5C-D). The translocation was balanced, resulting in 2 fusion chromosomes joining PD-L1 with the telomeric half and PD-L2 with the centromeric part of PIM1, respectively. In sample SL55, the location of the breakpoint in the PD-L1/PD-L2 locus was at the 3′ end of PD-L2 (supplemental Figure 5E). The translocation partner was IGH (Sμ), thus bringing the total number of PD-L1/PD-L2–IGH translocations to 2. In the rearrangements identified in samples 2168 and SL55, the entire PD-L1 and/or PD-L2 genes are being juxtaposed to their translocation partners and thus no fusion transcripts are generated (confirmed by RNAseq analysis). In one additional sample, SL24, breaks in the PD-L1/PD-L2 and IGH loci were identified by WGS, in accordance with the FISH data. However, we were not able to validate it by breakpoint-specific PCRs, probably because of DNA degradation in this sample. CNV analysis from the WGS data confirmed the amplification of the PD-L1 locus in sample 2171.
PD-L1/PD-L2 gene expression analysis
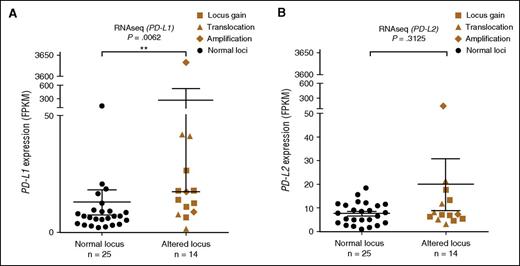
RNAseq was performed on 85 samples, including all samples that underwent WGS and together with cytogenetic analysis. The status of the PD-L1/PD-L2 locus was known in 39 samples that underwent RNAseq. The median expression level was 9.3 FPKM for PD-L1 and 7.4 FPKM for PD-L2. In 2 of the 3 samples with proposed translocation between IGH and PD-L1/PD-L2, DL48 and SL24, the expression of PD-L1 was increased to 41.1 and 41.7 FPKM, respectively. In sample DL509, where PD-L1 and TP63 were juxtaposed, the expression levels of both PD-L1 (13.8 FPKM) and TP63 (9.8 vs a median expression level of 2.2 FPKM) were increased. In sample 2168, where the translocation between the PD-L1/PD-L2 and PIM1 loci was observed, the expression of PD-L2 and PIM1 was upregulated (supplemental Figure 7). A significantly higher expression of PD-L1 in samples harboring cytogenetic alterations in the PD-L1/PD-L2 locus was furthermore observed (Mann-Whitney U test, P = .0062; Figure 3A). No association was found between PD-L2 expression and cytogenetic alterations (Figure 3B). High expression of PD-L1 or PD-L2 does not appear to coincide or be mutually exclusive. These results were furthermore validated by complementary qPCR in 166 samples from the Chinese cohort (data not shown).
mRNA expression data in relation to cytogenetic alterations. (A-B) Expression levels of PD-L1 and PD-L2 measured by RNAseq, presented in FPKM values. The Mann-Whitney U test was used to calculate statistical significance. **P < .01. The error bars represent standard error of the mean.
mRNA expression data in relation to cytogenetic alterations. (A-B) Expression levels of PD-L1 and PD-L2 measured by RNAseq, presented in FPKM values. The Mann-Whitney U test was used to calculate statistical significance. **P < .01. The error bars represent standard error of the mean.
PD-L1 and PD-L2 protein expression
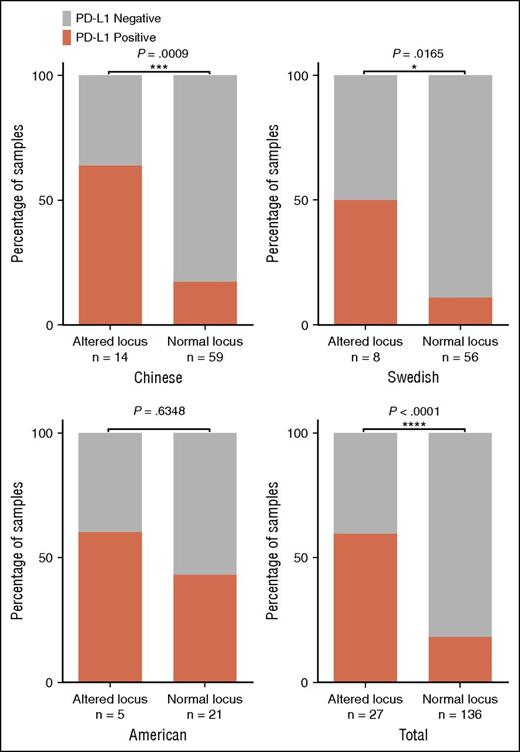
PD-L1 IHC analysis was performed in DLBCL samples derived from the American and Swedish cohorts as well as for 73 samples from the Chinese cohort. Forty-three of 163 (26.4%) of the assessed tumors were positive for PD-L1 expression, including all samples harboring translocations or amplifications in the PD-L1/PD-L2 locus. Notably, the protein levels were very high in sample SL55 carrying the translocation between the PD-L1/PD-L2 and IGH loci (supplemental Figure 8), whereas the mRNA levels of PD-L1 measured by RNAseq appeared to be low (supplemental Figure 7). This may reflect the low quality of RNA of this particular sample (prepared from FFPE). By pooling together all samples evaluated by both IHC and genetic/cytogenetic analyses, a strong association between PD-L1 protein expression and alterations in the PD-L1/PD-L2 locus was observed (Fisher exact test, P < .0001; Figure 4). Furthermore, the PD-L1 expression was more frequently observed in non-GCB compared with GCB samples (supplemental Figure 9). PD-L2 expression was assessed in 84 samples and, in concordance with RNA expression data, no association was observed between cytogenetic alterations and PD-L2 protein expression (supplemental Figure 10).
PD-L1 IHC on 163 DLBCLs. Correlation of PD-L1 protein expression and the occurrence of cytogenetic alterations in the different cohorts expressed as a percentage of the total number of samples in each column. Fisher exact test was used for statistical analysis. *P < .05, ***P < .001, ****P < .0001.
PD-L1 IHC on 163 DLBCLs. Correlation of PD-L1 protein expression and the occurrence of cytogenetic alterations in the different cohorts expressed as a percentage of the total number of samples in each column. Fisher exact test was used for statistical analysis. *P < .05, ***P < .001, ****P < .0001.
HLA class I expression
Because HLA class I expression is indispensable for antigenic recognition by the immune system, we assessed HLA class I expression in 94 DLBCL samples for which material was available. The prevalence of HLA class I loss was 45% in the Chinese, 44% in the American, and 36% in the Swedish-cohort. Notably, 4 of 5 samples with translocations or amplifications affecting PD-L1/PD-L2 were negative for HLA class I expression. However, no correlation was observed between the expression of HLA class I and PD-L1 across the cohorts. Loss of HLA class I expression in DLBCL is often associated with inactivation of the B2M gene, which encodes for the β chain of the HLA class I heterodimer.53,54 In 9 of the HLA class I–negative samples, we were able to cross the IHC data with RNA and genomic sequencing results and found that 7 of those samples either express B2M in levels lower than the sample median or harbor B2M mutations (identified by WGS), or both (supplemental Figure 11). This may explain the loss of HLA class I expression in these samples.
Discussion
We report here that genetic/cytogenetic aberrations involving the PD-L1/PD-L2 locus can be identified in ∼20% of DLBCLs and that these alterations occur mostly in the non-GCB subtype of this disease. We further demonstrated that, to the base pair resolution, PD-L1 is a novel fusion partner of IGH in DLBCL, whereas PIM1 and TP63 are previously unappreciated translocation partners for the PD-L1/PD-L2 locus. Samples with cytogenetic changes in the PD-L1/PD-L2 locus, especially those with translocations or amplifications, were more likely to overexpress PD-L1 at the mRNA and/or protein level. Previous reports of rearrangements in the PD-L1/PD-L2 locus were mainly focused on HL, PMBCL, PTL and PNSL.33-35 The latter 2 forms of large B-cell lymphomas are located in the immune privileged sites that are subjected to a different and unique immunologic context.55 Our study demonstrated the genetic basis for PD-L1 overexpression in DLBCL, not otherwise specified.
The expression of PD-L1 protein has previously been associated with rapid progression of disease in various cancers,56-58 including DLBCL.37 Our results are in general agreement with those reports and indicate that patients with a more aggressive form of disease (non-GCB) could potentially benefit from treatments targeting the PD-1–PD-L1/PD-L2 axis. Previous work also suggested that preexisting infiltration by CD8+ T cells is a prerequisite for a successful response to PD-1 blockade therapy.59 Furthermore, the presence of neoantigens resulting from an increased mutation load seems to enhance the T-cell infiltration.60,61 Moreover, studies from colorectal carcinoma showed that patients with impaired mismatch repair (MMR) respond better to the anti–PD-1 therapy.62 Although the mechanisms responsible for this relation are not clear, it is hypothesized that the improved responsiveness to immunotherapy is caused by an increase in the number of neoantigens as a result of the higher mutational load associated with the impaired MMR.25,63 Aberrant SHM as well as mutations in the MMR pathway contribute to an increase of the mutation load in DLBCL.9,52 Notably, sample DL48, where the original PD-L1–IGH translocation was identified, carries a somatic deleterious mutation in the MSH2 gene, a major component of MMR, and shows an increased number of somatic mutations in the coding genome.25 In addition, histologic examination confirmed CD8+ T-cell infiltration in the sample. Taken together, a subset of DLBCLs, represented by DL48, is likely to respond well to the anti–PD-1–PD-L1 therapy.
The effect of PD-1–PD-L1 blockade therapy is likely to be dependent on the expression of HLA class I molecules on the tumor cells, which are engaged by T-cell receptors on T cells. Lack of expression of the HLA class I thus often serves as a potent escape mechanism from the immune system.64 HLA class I IHC in our cohort demonstrated that nearly half of DLBCL samples, including those with PD-L1/PD-L2 translocations or amplifications, have lost the expression of HLA class I. This raises the question whether anti PD-1–PD-L1/PD-L2 therapies would produce any effect on tumor cells that lack HLA class I expression. Intriguingly, a recent study performed on HL demonstrated an impressive result, where the majority of patients showed lasting responses to anti–PD-1 treatment32 despite the loss of HLA class I being common in HL.65
Furthermore, we observed in our cohort that some tumors have used 2 immune evasion mechanisms, loss of HLA class I expression and PD-L1 overexpression, where the first should have already provided tumor cells with full protection from the cytotoxic T-cell killing. However, we must take into account that DLBCLs arise from B cells, which, as professional antigen-presenting cells, also express the HLA class II. The latter present antigenic determinants to CD4+ helper T cells, which act by stimulating the response by other immune cells including macrophages and B cells.66 Thus, the dynamics of PD-L1 expression in DLBCL could be different from that in most other tumor types because of the unique physiologic function of their normal B-cell counterparts. The loss of expression of HLA class II in DLBCL has been described and was associated with increased aggressiveness and reduced immunosurveillance.67 Thus, the upregulation of PD-L1, even in the absence of HLA class I, may further shield DLBCL from the immune system in an HLA class II–dependent manner.
Expression of PD-L1 protein was observed in all samples with translocations or amplifications. In a number of samples, however, we observed expression of the protein without any cytogenetic alterations, indicating that there are other underlying mechanisms leading to the expression of PD-L1 in DLBCL. An association between PD-L1 expression and an active Epstein-Barr virus (EBV) infection in malignant B cells has previously been reported.68 We have compared the RNA and protein expression levels of PD-L1 and PD-L2 between EBV-positive and EBV-negative samples and observed a trend for higher expression of PD-L1 protein in EBV-positive tumors (supplemental Figure 12). Among the 24 samples from which both EBV infection information and PD-L1 IHC data were available, 9 were positive for PD-L1, of which 4 harbored cytogenetic alterations. Locus status information was lacking for 1 sample, but among the 4 with normal PD-L1/PD-L2 locus, only 1 case was EBV-positive. Therefore, although EBV infection can explain the expression of PD-L1 in some cases, it does not account for all the positive cases without cytogenetic alterations.
The cytogenetic alterations as well as protein expression of PD-L1/PD-L2 were more frequent in the non-GCB subtype. Non-malignant B cells require activation to express PD-L1.69 Previous studies suggest that genes/pathways expressed on non-GCB samples show a similarity to those in activated B cells,2,69 thus these conditions may be favorable for the constitutive expression of PD-L1. Stimulation of PD-L1 expression in non-GCB DLBCL can also be the result of active JAK/STAT3 signaling.70 MYD88 mutations have been found in ∼30% of non-GCB cases and they are associated with the constitutive expression and activation of the JAK2 kinase that in turn stimulates the expression of PD-L1.71,72 Regardless of the mechanism, the preference of expression of PD-L1 in the non-GCB/ABC disease subtype suggests that immunotherapies blocking PD-1 and PD-L1 may be promising in patients with this aggressive subtype of disease. Of note, we also attempted to classify our samples into the GCB and ABC disease subtypes by RNAseq based on the set of genes described previously.45 However, approximately one quarter of the samples could not be grouped to either GCB or ABC, because they displayed an intermediate expression pattern (supplemental Figure 13A), suggesting that the current classification for DLBCL might still be oversimplified. In addition, we also observed a distinctive RNA expression signature in tumors overexpressing PD-L1, which might also reflect an altered tumor microenvironment of these tumors (supplemental Figure 13B). Further integration of genomic, transcriptomic, and clinical data would be expected to provide us a more comprehensive picture of DLBCL.
In summary, our data indicate that genetic alterations affecting the PD-L1/PD-L2 locus, especially translocations and amplification, lead to the overexpression of the immune-modulatory factor PD-L1. In addition, our data suggest that patients with an aggressive subtype of disease may benefit from therapies blocking the PD-1–PD-L1/PD-L2 interaction. However, the factors that can predict the efficacy of these therapies are still not fully elucidated.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank S. Lagercrantz for kindly providing the tissue material for FISH optimization, and A. Zaravinos for techinical help.
This work was supported by the Swedish Cancer Society, the Swedish Research Council, the European Research Council, the Swedish Children Cancer Fund and National Natural Science Foundation of China (grants 81302045 and 81572590).
Authorship
Contribution: K.G. prepared samples; performed cytogenetic analysis, Sanger sequencing, qPCR, and IHC; collected, analyzed, and interpreted the data; and wrote the manuscript; L.C. analyzed bioinformatics; M.B. acquired and prepared samples and performed IHC analysis; N.F.C.C.d.M. performed IHC analysis and helped write the manuscript; S.L. performed the cytogenetic analysis; M.F. performed IHC analysis and prepared samples; W.R., W.F., L.Z., Y.Z., X.W., H.Z., G.B., G.E., R.P., R.D.-F., and L.P. prepared samples and/or the collection of clinical information; B.M. contributed to data analyses; S.Z., Y.H., and K.W. performed WGS analysis; C.S. performed IHC analysis; M.N. and M.R.T. supervised the cytogenetic analysis; and Q.P.-H. designed and supervised the study, interpreted the data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Qiang Pan-Hammarström, Department of Laboratory Medicine, Karolinska Institutet, 14186 Stockholm, Sweden; e-mail: qiang.pan-hammarstrom@ki.se; Roujun Peng, State Key Laboratory of Oncology in South China and Department of Medical Oncology, Sun Yat-Sen University Cancer Center, Guangzhou, China; e-mail: pengrj@sysucc.org.cn; and Huilai Zhang, Department of Lymphoma, Tianjin Medical University Cancer Institute and Hospital, National Clinical Research Center of Cancer, Key Laboratory of Cancer Prevention and Therapy, Tianjin, China; e-mail: zhlwgq@126.com.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal