In this issue of Blood, Anderson et al demonstrate that venetoclax induces apoptosis of chronic lymphocytic leukemia (CLL) cells independently of TP53 function in vitro and in vivo and suggest a role for BH3 profiling in determining a patient’s response to treatment.1
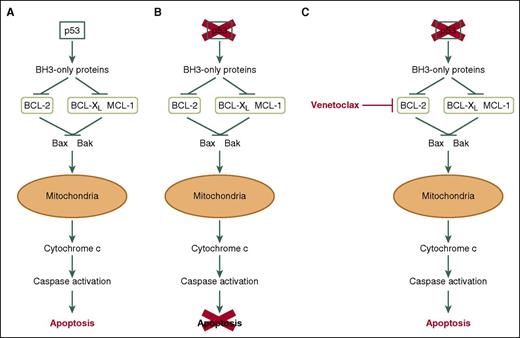
Venetoclax induces apoptosis of CLL cells independently of p53. (A) In response to DNA damage, the commonly mutated/deleted tumor suppressor p53 initiates apoptosis via the intrinsic apoptosis pathway. (B) TP53 mutation and/or deletion results in defective apoptosis in response to DNA damage and contributes to chemotherapy resistance. (C) Venetoclax inhibits BCL2, thereby inducing apoptosis of CLL cells in a mechanism that Anderson et al demonstrate to be independent of p53 expression/function in vitro and in vivo.
Venetoclax induces apoptosis of CLL cells independently of p53. (A) In response to DNA damage, the commonly mutated/deleted tumor suppressor p53 initiates apoptosis via the intrinsic apoptosis pathway. (B) TP53 mutation and/or deletion results in defective apoptosis in response to DNA damage and contributes to chemotherapy resistance. (C) Venetoclax inhibits BCL2, thereby inducing apoptosis of CLL cells in a mechanism that Anderson et al demonstrate to be independent of p53 expression/function in vitro and in vivo.
Apoptosis, a form of controlled programmed cell death, occurs following irreparable cellular damage. The Bcl-2-family of proteins consist of 3 main subfamilies that tightly control this apoptotic process. The first are antiapoptotic proteins such as B-cell lymphoma 2 (BCL-2), BCL-2–related gene, long isoform (BCL-XL), and myeloid cell leukemia 1 (MCL-1). The second are proapoptotic effector proteins including BCL-2 antagonist killer 1 (BAK) and BCL-2–associated x protein (BAX), and the third are proapoptotic BH3-only proteins such as BCL-2–associated agonist of cell death (BAD), phorbol-12-myristate-13-acetate-induced protein 1 (PMAIP1/Noxa), BH3-interacting domain death agonist (BID), and BCL-2–interacting mediator of cell death (BIM).2 Inhibition of antiapoptotic proteins by the proapoptotic BH3-only proteins results in BAX/BAK dimerization and insertion into the mitochondrial membrane where they initiate mitochondrial outer membrane permeabilization (MOMP). MOMP results in the release of cytochrome C, caspase activation, and, subsequently, apoptosis2 (see figure panel A). CLL cells have impaired apoptosis due to overexpression of prosurvival BCL-2, resulting in part from deletion of microRNA15/16-1 on chromosome 13 [del(13q)]. However their reliance and addiction to BCL-2 overexpression leaves them susceptible to therapeutic targeting. A number of the proapoptotic BH3-only proteins are induced by p53; therefore, del(17p)/TP53 mutations can impair basal and drug-induced apoptosis that requires functional p53 (see figure panel B). However, if we mimic these p53-induced BH3-only proteins, we can potentially induce cellular apoptosis regardless of p53 functionality (see figure panel C). Drugs like venetoclax (ABT-199) mimic the BH3 domain of BAD and inhibit BCL-2 function (inhibitory concentration [IC]50 <0.01 nM [inhibitory constant (Ki)]), with reduced or no inhibition of BCL-XL (IC50 48 nM [Ki]) and MCL-1 (>444 nM [Ki]),3 respectively, resulting in apoptosis. Venetoclax is approved for the treatment of CLL patients with del(17p)4 and is currently in phase 1b/2 clinical trials alone (#NCT02265731) and in combination with bendamustine, duvelisib, ibrutinib, and antibody therapies (#NCT02640833, #NCT01685892, #NCT01671904, and #NCT02427451).
Here in conjunction with the phase 1 clinical trial (M12-175), Anderson et al show venetoclax induces rapid apoptosis of CLL cells, in vitro, in paired peripheral blood and bone marrow samples in a caspase-dependent manner, at similar concentrations. However, this might be expected, because the bone marrow–derived tumor cells were evaluated following density gradient separation and therefore lacked support from signals within the tissue microenvironment. Importantly, they showed venetoclax kills CLL cells in the presence or absence of del(17p) using primary human CLL cells, B cells isolated from mice lacking TP53 expression (Trp53−/−), and in a human B cell line with clustered regularly interspaced short palindromic repeats/Cas9-mediated TP53 loss, highlighting that BH3-mimetics such as venetoclax can promote apoptosis in the absence of functional p53. Moreover, the authors showed that patients with the del(17p)/TP53 mutation did not differ in their initial apoptotic response to venetoclax in vivo, compared with patients with functional p53. This is seemingly in contrast with reports which indicated that venetoclax-induced progression-free survival (PFS) was poorer in patients with del(17p) compared with patients without del(17p),5 indicating that over a longer treatment period, del(17p) abnormalities may adversely impact venetoclax-mediated PFS, irrespective of the depth of initial response to treatment. However, this may simply reflect evolution of samples with a complex karyotype, the involvement of genes other than TP53 in patients with del(17p), or because of signals within the tumor microenvironment. In the present study, sensitivity of tumor cells from CLL lymph nodes to venetoclax was not evaluated. However, it is likely that microenvironmental signals within the lymph node will determine how well a patient responds to treatment, because they can protect tumor cells from therapy-induced killing. CLL cells within the lymph node microenvironment express greater levels of MCL-1 and BCL-XL protein compared with peripheral blood.6 Both of these proteins are induced in vitro following treatment with anti-immunoglobulin M or interleukin-4 (IL-4)/CD154, mimicking signals within the lymph node microenvironment from (auto)antigen and T cells, respectively.7-10 Expression of these proteins and particularly MCL-1 confers resistance to venetoclax-induced killing in vitro.7,8 Indeed, MCL-1 levels vary between CLL samples9 ; therefore, its expression before and after venetoclax treatment may identify those patients who are likely to progress and warrants further investigation as a predictive marker.
Anderson et al investigated in vitro sensitivity to venetoclax-induced apoptosis with the depth of clinical response in patients; however, there was no correlation. Whereas, BH3 profiling, as a measure of mitochondrial priming/depolarization by a BIM peptide in vitro, did correlate with a reduction in the circulating lymphocyte count and bone marrow tumor burden by venetoclax. However there was no correlation with deeper lymph node responses. This suggests that BH3 profiling may in part be beneficial in determining a patient’s response to venetoclax and perhaps other BH3 mimetics. However, it will be important to determine whether the depth of response observed with venetoclax in this study correlates with improved PFS and overall survival with longer follow-up before we can understand the potential benefit of using BIM BH3 profiling to better predict patient outcome. Importantly, performing BH3 profiling instead on CLL cells from the lymph node or after B-cell receptor (BCR) signaling, IL-4/CD154 treatment, or with stromal support may provide better insight into BH3 profiling as a predictive biomarker in determining a patient’s sensitivity to venetoclax. Furthermore, these data support a strategy for simultaneous treatment with brutons tyrosine kinase or spleen tyrosine kinase inhibitors, which inhibit ingress into and/or promote efflux out of the lymph nodes into the blood, and venetoclax.
Despite these unresolved queries, Anderson et al provide compelling new data and insight into the biology of venetoclax for the treatment of CLL. Since a proportion of patients are already developing resistance to BCR kinase inhibitors and venetoclax as a single agent has been shown to induce minimum residual disease negativity in 5% of patients studied,5 venetoclax alone or in combination with BCR kinase inhibitors may provide an important therapeutic option for this currently incurable disease.
Conflict-of-interest disclosure: The authors declare no competing financial interests.