Key Points
Vaccination using WT1 peptides and dendritic cells induced WT1-specific human CTLs in vivo in HLA class I Tg NSG mice.
Human HSCs transduced with human TCR genes generated HLA class I–restricted, WT1-specific CTLs in vivo.
Abstract
Induction of specific immune response against therapy-resistant tumor cells can potentially improve clinical outcomes in malignancies. To optimize immunotherapy in the clinic, we aimed to create an in vivo model enabling us to analyze human cytotoxic T-lymphocyte (CTL) responses against human malignancies. To this end, we developed NOD/SCID/IL2rgKO (NSG) mice expressing the HLA class I molecules HLA-A*0201 and A*2402. In the bone marrow (BM) and spleen of HLA class I transgenic (Tg) NSG mice transplanted with cord blood hematopoietic stem cells (HSCs), we found human memory CD8+ T cells and antigen-presenting cells. To evaluate antigen-specific human CTL responses, we immunized HLA class I Tg NSG mice using polyinosinic:polycytidylic acid mixed Wilms tumor 1 (WT1) peptides, with or without WT1 peptide–loaded autologous dendritic cells. After immunization, the frequencies of HLA-restricted WT1-specific CTLs increased significantly in the spleen. Next, we transplanted the WT1-specific T-cell receptor (WT1-TCR) gene–transduced human HSCs into HLA class I Tg NSG newborn mice. WT1 tetramer-positive CD8+ T cells differentiated from WT1-TCR-transduced HSCs in the recipients’ BM, spleen, and thymus. Upon stimulation with WT1 peptide in vitro, these CTLs produced interferon-γ and showed lytic activity against leukemia cells in an antigen-specific, HLA-restricted manner. HLA class I Tg NSG xenografts may serve as a preclinical model to develop effective immunotherapy against human malignancies.
Introduction
The immune system prevents infectious disease initiation and progression and functions in multiple homeostatic processes. However, dysfunctional immunity is observed in patients with malignancies, contributing to neoplastic progression. Therefore, reconstitution of immunity by allogeneic stem cell transplant or activation of specific and nonspecific immunity-targeting diseases improves clinical outcomes in patients with solid cancers and in those with hematologic malignancies.1,2 Such treatment can be carried out by vaccination and by adoptive immunotherapy.
Vaccinations aim to elicit antigen-specific effector cell–mediated immune responses in vivo.3 Among several candidates, peptide vaccines and dendritic cell (DC) vaccines were 2 widely selected protocols. In the last 2 decades, however, administration of these vaccines has not significantly improved the prognosis of patients with solid cancers including melanoma and other types of solid tumors.4,5 Although several recent trials reported encouraging clinical outcomes using glycoprotein 100 peptides in combination with interleukin (IL)-2 for the treatment of melanoma,6 or patient-derived antigen-presenting cells (APCs; sipuleucel-T) for the treatment of prostate cancer,7 cancer vaccination appears to require modifications based on increased understanding of in vivo biology of human APCs and T cells.
In contrast, immunotherapy based on adoptive transfer of ex vivo expanded tumor-reactive T cells has achieved promising results. In metastatic melanoma, adoptive transfer of tumor-infiltrating lymphocytes in combination with chemotherapy or irradiation has improved cure rates up to 20% to 40%.8 Because the antitumor effect of tumor-infiltrating lymphocytes has not been confirmed in malignancies other than melanoma, genetically engineered T cells that express tumor antigen–specific T-cell receptor (TCR) genes or chimeric antigen receptors have been developed.9 Recent clinical trials showed improved clinical outcomes in patients treated with genetically engineered T cells,10-13 whereas adverse effects were observed immediately after the transfusion of T cells expressing chimeric antigen receptors.14,15
In several clinical trials of vaccination therapies for hematologic malignancies, promising responses were observed using various antigens, including proteinase 316 and Wilms tumor 1 (WT1)16,17 for acute myeloid leukemia (AML), breakpoint cluster region/Abelson murine leukemia for chronic myelogenous leukemia,18 and patient-specific idiotypes derived from malignant B-cell clones for follicular lymphoma.19 In particular, for patients with poor prognostic factors, development of immunotherapy targeting minimal residual disease or leukemia stem cells (LSCs) should play an essential role in achieving long-term patient survival.
We recently reported that WT1, a transcription factor expressed in variety of malignant tissues, is highly expressed by CD34+CD38− AML cells.20 WT1 is considered one of the best antigens to be used for immunotherapy against malignancies, based on multiple criteria such as therapeutic function, immunogenicity, and specificity.21 Using WT1 peptide or full-length messenger (m)RNA for WT1, clinical trials against hematologic malignancies detected increased frequencies of WT1-specific CD8+ T cells in patient blood after the treatment.16,17,22,23 Nevertheless, to accomplish significant improvement in clinical outcomes of AML patients, we need to better understand the biology of the human immune system leading to efficient activation of human acquired immunity against tumor antigens.
In the present study, we aimed to develop an in vivo system for induction of antigen-specific, HLA-restricted human CD8+ T cells after vaccination. HLA class I–expressing NOD/SCID/IL2rgKO (NSG) mice supported the development of human T cells and APCs after engraftment with human cord blood (CB) HSCs. We detected high frequencies of WT1-specific CD8+ T cells in the bone marrow (BM) and spleen of HLA class I–expressing NSG mice after vaccination. Moreover, we confirmed the development of WT1-specific CD8+ T cells in vivo after engraftment of human HSCs transduced with WT1-specific TCR (WT1-TCR) Vα and Vβ genes. The antigen-specific human CD8+ T cells expanded in response to WT1 antigen and were functional both in cytokine production and cytotoxicity. Development of immunotherapy protocols in HLA class I–expressing NSG mice may facilitate the development and optimization of antigen-specific immunotherapy against malignancies.
Materials and methods
Detailed experimental methods are described in supplemental Methods, available on the Blood Web site.
Flow cytometry
For phenotypical analysis, cells labeled with monoclonal antibodies (mAbs) (supplemental Table 1) were analyzed using FACSAria and FACSCanto II instruments (BD Biosciences).
Immunization of humanized mice
In the DC vaccine group, HSC-reconstituted HLA class I transgenic (Tg) NSG recipients were injected with WT1 peptide–loaded DCs via retro-orbital plexus on day 0, followed by intraperitoneal and subcutaneous immunization with 300 μg of WT1126-134 or WT1235-243 synthetic peptide mixed with 150 μg of polyinosinic:polycytidylic acid (poly I:C; InvivoGen) on days 0, 10, and 20. In the vaccine group without DCs, the same doses of WT1126-134 or WT1235-243 synthetic peptide mixed with poly I:C were administered intraperitoneally and subcutaneously on days 0, 10, and 20. One week after the last immunization, mice were necropsied for evaluation of human immune cells in peripheral blood (PB), BM, spleen, lymph nodes, and thymus.
In vitro expansion of human CD8+ T cells derived from mice engrafted with human HSCs
Human cytotoxic T-lymphocytes (CTLs) were expanded from HLA class I Tg NSG humanized mice as described previously, with some modifications.24 Splenocytes, thymocytes, and BM cells from recipient mice were stimulated with irradiated autologous lymphoblastoid cell lines (LCLs) pulsed with 40 μg/mL of WT1126-134 or WT1235-243 synthetic peptide at a responder-to-stimulator ratio of 1:1.
Lentiviral transduction
Purified CB CD34+CD38− cells were incubated with concentrated lentiviral supernatant at a multiplicity of infection of 100 to 200. After 5 days of incubation, green fluorescent protein–expressing (GFP+) cells were sorted using a FACSAria cell sorter (BD Biosciences) and transplanted into irradiated HLA class I Tg NSG newborn mice.
ELISpot assay
WT1-specific T-cell responses were quantified by the interferon (IFN)-γ enzyme-linked immunospot (ELISpot; Mabtech) assay as described previously.24 Pictures of each well were captured using AlphaImager Image Analysis System (Alpha Innotech), and individual spots were counted per well. Using the same protocol as for the IFN-γ assay, the production of human tumor necrosis factor α by human WT1-specific CD8+ T cells was also confirmed by ELISpot assay.
51Cr release assay
Cytotoxic activity of WT1-specific CTLs was evaluated by chromium 51 (51Cr) release assay as described previously.24 To examine the HLA-restricted cytotoxicity, target cells were incubated with anti-HLA class I framework mAb (w6/32; American Type Culture Collection) or anti-HLA-DR mAb (L243; American Type Culture Collection) at 10 μg/mL for 1 hour, followed by adding the effector cells. The percentage of specific lysis was calculated as (experimental release cpm − spontaneous release cpm)/(maximal release cpm − spontaneous release cpm) × 100.
In vivo cytotoxicity assay
HLA-A24–expressing K562 cells25 were cultured with or without WT1-specific CTLs for 5 hours. After harvesting all the cultured cells, the cells were inoculated subcutaneously into female NSG mice. WT1-specific CTLs were adoptively transferred weekly. At 24 to 28 days postinoculation, the mice were euthanized to evaluate tumor size and weight.
Analysis of TCR repertoire of CTLs
After in vitro expansion, WT1-specific CTLs were purified by fluorescence-activated cell sorting (FACS). As a control, 2.4 × 104 to 1.5 × 105 human CD45+CD3+CD8+ cells were purified from spleens of NSG mice engrafted with nontransduced human HSCs. Total RNA was extracted using TRIzol reagent (Invitrogen) and reverse transcribed for synthesis of first-strand complementary DNA using the SMARTer RACE cDNA Amplification Kit (Clontech). Both universal mix primer and primers specific for the TCRα or TCRβ constant region sequence were used for 5′ end–specific polymerase chain reaction (PCR) amplifications, resulting in both TCRα and TCRβ PCR products of high purity that were then sequenced using MiSeq (Illumina). All reads of the TCRα and TCRβ repertoire sequences were analyzed using Perl scripts based on the USEARCH algorithm (http://drive5.com/usearch/). V-region consensus sequences in each cluster were searched on the ImMunoGeneTics sites (www.imgt.org/IMGT_vquest/share/textes/).
Results
Human CB HSCs generate both human T cells and APCs in HLA class I Tg NSG mice
We developed immunodeficient NSG mice expressing HLA class I antigens by backcrossing HLA-A*020124 or HLA-A*2402 transgenes onto the NSG background. Both transgenes also encode covalently bound human β-2-microglobulin. By reverse-transcription PCR, HLA transgenes were detected in the BM, spleen, and CD45−EpCAM+ thymic epithelial cells derived from both NSG-HLA-A2/HHD mice and NSG-HLA-A24/HHD mice (Figure 1A). We then transplanted 4.5 × 103 to 5.6 × 104 purified human HLA-matched CD3−CD4−CD8−CD34+CD38− CB HSCs into HLA class I Tg NSG newborn mice. At 15 to 37 weeks posttransplant, we confirmed engraftment of human (h)CD45+ leukocytes in the PB, BM, spleen, mesenteric lymph nodes (MLNs), axillary lymph nodes, and thymus (Figure 1B-C; Table 1; PB, 72.7% ± 6.9%; spleen, 91.5% ± 2.0%; BM, 72.4% ± 9.2%; MLN, 97.3% ± 0.7%; and thymus, 97.6% ± 1.2% [n = 11]; axillary lymph nodes, 96.7% ± 1.4 % [n = 10]). Human CD3+ T cells were 65.9% ± 7.2% of engrafted hCD45+ leukocytes in the recipient spleen. CD4+CD8+ double-positive T cells accounted for 34.2% ± 12.5% in the thymus (n = 6), whereas CD4+ or CD8+ single-positive T cells were 26.2% ± 5.9% or 31.7% ± 11.3%, respectively, of engrafted human CD3+ T cells. Within the human CD8+ fraction, we identified CD45RA+CCR7+ naïve, CD45RA−CCR7+ central memory (CM), CD45RA−CCR7− effector memory (EM), and CD45RA+CCR7− terminally differentiated (EMRA) T-cell subsets in the recipient spleen (Figure 1D-E; naïve, 20.8% ± 10.0%; CM, 13.7% ± 4.9%; EM, 60.9% ± 10.3%; and EMRA, 4.9% ± 1.8% [n = 10]). Cytoplasmic expression of granzyme A was the highest in EM CD8+ T cells (Figure 1F; supplemental Figure 1; naïve, 10.0% ± 2.1%; CM, 42.0% ± 8.9%; EM, 75.4% ± 10.7%; and EMRA, 55.1% ± 10.3% [n = 4]).
HLA expression and multilineage human immune subsets in HLA class I Tg NSG humanized mice. (A) Reverse-transcription PCR for HLA-A*0201, HLA-A*2402, human β-2-microglobulin (hu-β2M), and murine β-2-microglobulin (ms-β2M) using complementary DNA from BM, spleen, and msCD45−EpCAM+ thymic epithelial cells (TEC) derived from nonengrafted mice. “A2Tg” (left) and “A24Tg” (right) indicate NSG-HLA-A2/HHD and NSG-HLA-A24/HHD, respectively. (B) Representative contour plots indicating the engraftment of hCD45+ leukocytes, hCD3+ T cells, and hCD19+ B cells in the spleen of an NSG-HLA-A24/HHD recipient at 18 weeks after transplant. (C) Engraftment levels of hCD45+ cells in each organ (n = 11 for PB, spleen, BM, MLN, and thymus; n = 10 for axillary lymph nodes [AxLN]). (D) In the CD8+ cell fraction of the spleen of HLA class I Tg NSG recipients, naïve, CM, EM, and EMRA T-cell subsets were identified. (E) The frequencies of CD8+ memory T-cell subsets in the spleen of HLA class I Tg NSG recipients (n = 10). (F) Cytoplasmic expression of granzyme A in CD8+ T-cell subsets from naïve to E phenotype in the spleen of HLA class I Tg NSG recipients (n = 4). (G) Representative contour plots of human myeloid subsets in the BM of an HLA class I Tg NSG recipient. (H) The frequencies of monocytes (Mono; CD14+CD33+HLA-DR+), pDCs (CD123+CD11c−), and cDCs (CD33+HLA-DR+CD11c+) among hCD45+ cells in BM (left) and spleen (right). (I) cDCs were further divided into BDCA1+ and BDCA3+ DCs in BM (n = 8) (left) and spleen (n = 7) (right). Bars indicate the mean value. E, effector; ms, mouse; Spl, spleen; Thy, thymus.
HLA expression and multilineage human immune subsets in HLA class I Tg NSG humanized mice. (A) Reverse-transcription PCR for HLA-A*0201, HLA-A*2402, human β-2-microglobulin (hu-β2M), and murine β-2-microglobulin (ms-β2M) using complementary DNA from BM, spleen, and msCD45−EpCAM+ thymic epithelial cells (TEC) derived from nonengrafted mice. “A2Tg” (left) and “A24Tg” (right) indicate NSG-HLA-A2/HHD and NSG-HLA-A24/HHD, respectively. (B) Representative contour plots indicating the engraftment of hCD45+ leukocytes, hCD3+ T cells, and hCD19+ B cells in the spleen of an NSG-HLA-A24/HHD recipient at 18 weeks after transplant. (C) Engraftment levels of hCD45+ cells in each organ (n = 11 for PB, spleen, BM, MLN, and thymus; n = 10 for axillary lymph nodes [AxLN]). (D) In the CD8+ cell fraction of the spleen of HLA class I Tg NSG recipients, naïve, CM, EM, and EMRA T-cell subsets were identified. (E) The frequencies of CD8+ memory T-cell subsets in the spleen of HLA class I Tg NSG recipients (n = 10). (F) Cytoplasmic expression of granzyme A in CD8+ T-cell subsets from naïve to E phenotype in the spleen of HLA class I Tg NSG recipients (n = 4). (G) Representative contour plots of human myeloid subsets in the BM of an HLA class I Tg NSG recipient. (H) The frequencies of monocytes (Mono; CD14+CD33+HLA-DR+), pDCs (CD123+CD11c−), and cDCs (CD33+HLA-DR+CD11c+) among hCD45+ cells in BM (left) and spleen (right). (I) cDCs were further divided into BDCA1+ and BDCA3+ DCs in BM (n = 8) (left) and spleen (n = 7) (right). Bars indicate the mean value. E, effector; ms, mouse; Spl, spleen; Thy, thymus.
Characteristics of HLA class I Tg NSG recipients
| Immunization group recipient ID . | CB ID . | HLA . | PB chimerism, % . | Spl chimerism, % . | Spl CD3+/hCD45+, % . | Spl CD33+/hCD45+, % . | Spl WT1 tet+/CD8+, % . |
|---|---|---|---|---|---|---|---|
| No immunization (n = 10) | |||||||
| A2-1 | 1-030304 | A2+A24+* | 39.6 | 92.2 | 72.4 | 4.2 | 0.039 |
| A2-2 | 23-4 | A0201/0206 | 93.4 | 94.0 | 57.4 | 4.3 | 0.015 |
| A2-3 | 23-35 | A0201/0206 | 60.1 | 84.1 | 89.2 | 2.4 | 0.000 |
| A2-4 | 23-35 | A0201/0206 | 65.7 | 77.1 | 84.4 | 2.0 | 0.029 |
| A24-1 | 22-52 | A2−A24+* | 96.1 | 99.1 | 81.0 | 1.5 | 0.062 |
| A24-2 | 22-58 | A0206/2402 | 76.1 | 98.3 | 69.2 | 2.7 | 0.071 |
| A24-3 | 23-3 | A0206/2402 | 81.2 | 92.4 | 39.3 | 4.6 | 0.061 |
| A24-4 | 23-11 | A1101/2402 | 94.6 | 98.5 | 87.7 | 1.3 | 0.045 |
| A24-5 | 24-13 | A2402/− | 64.8 | 88.8 | 19.2 | 2.5 | 0.048 |
| A24-6 | 24-19 | A2402/3303 | 30.9 | 87.9 | 59.7 | 4.2 | 0.041 |
| Mean ± SE | 70.3 ± 7.1 | 91.2 ± 2.2 | 65.9 ± 7.2 | 3.0 ± 0.4 | 0.041 ± 0.007 | ||
| Immunized without DCs (n = 10) | |||||||
| A2-5 | 20-71 | A0206/2402 | 23.7 | NA | NA | NA | 0.208 |
| A2-6 | 22-7 | A2+A24−* | 78.9 | 95.2 | 58.5 | 5.3 | 0.050 |
| A2-7 | 22-18 | A2+A24+* | 97.7 | 99.2 | 64.4 | 10.6 | 0.030 |
| A24-7 | 22-58 | A0206/2402 | 47.1 | 94.5 | 69.8 | 3.8 | 0.353 |
| A24-8 | 22-31 | A2402/3201 | 75.2 | 75.4 | 79.1 | 1.5 | 0.116 |
| A24-9 | 22-29 | A2−A24+* | 44.1 | 89.8 | 36.5 | 4.7 | 0.022 |
| A24-10 | 23-3 | A0206/2402 | 63.5 | 89.8 | 22.5 | 4.3 | 0.035 |
| A24-11 | 23-3 | A0206/2402 | 50.1 | 74.7 | 14.7 | 5.5 | 0.287 |
| A24-12 | 23-11 | A1101/2402 | 15.1 | 62.7 | 39.3 | 4.5 | 0.252 |
| A24-13 | 24-13 | A2402/− | 58.0 | 92.6 | 13.5 | 1.6 | 0.349 |
| Mean ± SE | 55.3 ± 7.9 | 86.0 ± 4.1 | 44.2 ± 8.2 | 4.7 ± 0.9 | 0.170 ± 0.043 | ||
| Immunized with DCs (n = 9) | |||||||
| A2-8 | 20-28 | A0201/3101 | 55.8 | NA | NA | NA | 0.503 |
| A2-9 | 07-329 | A0202/3303 | 58.9 | NA | NA | NA | 0.072 |
| A2-10 | 22-63 | A2+A24−* | 53.8 | 79.4 | 44.2 | 3.3 | 0.213 |
| A24-14 | 22-52 | A2−A24+* | 66.2 | 92.5 | 72.6 | 4.1 | 0.201 |
| A24-15 | 22-29 | A2−A24+* | 70.2 | 93.5 | 5.9 | 7.0 | 0.334 |
| A24-16 | 23-3 | A0206/2402 | 39.3 | 83.1 | 13.8 | 1.6 | 0.248 |
| A24-17 | 23-3 | A0206/2402 | 84.5 | 87.1 | 78.4 | 3.8 | 0.056 |
| A24-18 | 23-16 | A2402/3303 | 93.6 | 95.6 | 93.0 | 0.9 | 0.218 |
| A24-19 | 24-13 | A2402/− | 81.9 | 94.9 | 11.9 | 1.0 | 0.222 |
| Mean ± SE | 61.2 ± 8.3 | 78.8 ± 10.3 | 41.0 ± 12.0 | 2.8 ± 0.7 | 0.211 ± 0.046 |
| Immunization group recipient ID . | CB ID . | HLA . | PB chimerism, % . | Spl chimerism, % . | Spl CD3+/hCD45+, % . | Spl CD33+/hCD45+, % . | Spl WT1 tet+/CD8+, % . |
|---|---|---|---|---|---|---|---|
| No immunization (n = 10) | |||||||
| A2-1 | 1-030304 | A2+A24+* | 39.6 | 92.2 | 72.4 | 4.2 | 0.039 |
| A2-2 | 23-4 | A0201/0206 | 93.4 | 94.0 | 57.4 | 4.3 | 0.015 |
| A2-3 | 23-35 | A0201/0206 | 60.1 | 84.1 | 89.2 | 2.4 | 0.000 |
| A2-4 | 23-35 | A0201/0206 | 65.7 | 77.1 | 84.4 | 2.0 | 0.029 |
| A24-1 | 22-52 | A2−A24+* | 96.1 | 99.1 | 81.0 | 1.5 | 0.062 |
| A24-2 | 22-58 | A0206/2402 | 76.1 | 98.3 | 69.2 | 2.7 | 0.071 |
| A24-3 | 23-3 | A0206/2402 | 81.2 | 92.4 | 39.3 | 4.6 | 0.061 |
| A24-4 | 23-11 | A1101/2402 | 94.6 | 98.5 | 87.7 | 1.3 | 0.045 |
| A24-5 | 24-13 | A2402/− | 64.8 | 88.8 | 19.2 | 2.5 | 0.048 |
| A24-6 | 24-19 | A2402/3303 | 30.9 | 87.9 | 59.7 | 4.2 | 0.041 |
| Mean ± SE | 70.3 ± 7.1 | 91.2 ± 2.2 | 65.9 ± 7.2 | 3.0 ± 0.4 | 0.041 ± 0.007 | ||
| Immunized without DCs (n = 10) | |||||||
| A2-5 | 20-71 | A0206/2402 | 23.7 | NA | NA | NA | 0.208 |
| A2-6 | 22-7 | A2+A24−* | 78.9 | 95.2 | 58.5 | 5.3 | 0.050 |
| A2-7 | 22-18 | A2+A24+* | 97.7 | 99.2 | 64.4 | 10.6 | 0.030 |
| A24-7 | 22-58 | A0206/2402 | 47.1 | 94.5 | 69.8 | 3.8 | 0.353 |
| A24-8 | 22-31 | A2402/3201 | 75.2 | 75.4 | 79.1 | 1.5 | 0.116 |
| A24-9 | 22-29 | A2−A24+* | 44.1 | 89.8 | 36.5 | 4.7 | 0.022 |
| A24-10 | 23-3 | A0206/2402 | 63.5 | 89.8 | 22.5 | 4.3 | 0.035 |
| A24-11 | 23-3 | A0206/2402 | 50.1 | 74.7 | 14.7 | 5.5 | 0.287 |
| A24-12 | 23-11 | A1101/2402 | 15.1 | 62.7 | 39.3 | 4.5 | 0.252 |
| A24-13 | 24-13 | A2402/− | 58.0 | 92.6 | 13.5 | 1.6 | 0.349 |
| Mean ± SE | 55.3 ± 7.9 | 86.0 ± 4.1 | 44.2 ± 8.2 | 4.7 ± 0.9 | 0.170 ± 0.043 | ||
| Immunized with DCs (n = 9) | |||||||
| A2-8 | 20-28 | A0201/3101 | 55.8 | NA | NA | NA | 0.503 |
| A2-9 | 07-329 | A0202/3303 | 58.9 | NA | NA | NA | 0.072 |
| A2-10 | 22-63 | A2+A24−* | 53.8 | 79.4 | 44.2 | 3.3 | 0.213 |
| A24-14 | 22-52 | A2−A24+* | 66.2 | 92.5 | 72.6 | 4.1 | 0.201 |
| A24-15 | 22-29 | A2−A24+* | 70.2 | 93.5 | 5.9 | 7.0 | 0.334 |
| A24-16 | 23-3 | A0206/2402 | 39.3 | 83.1 | 13.8 | 1.6 | 0.248 |
| A24-17 | 23-3 | A0206/2402 | 84.5 | 87.1 | 78.4 | 3.8 | 0.056 |
| A24-18 | 23-16 | A2402/3303 | 93.6 | 95.6 | 93.0 | 0.9 | 0.218 |
| A24-19 | 24-13 | A2402/− | 81.9 | 94.9 | 11.9 | 1.0 | 0.222 |
| Mean ± SE | 61.2 ± 8.3 | 78.8 ± 10.3 | 41.0 ± 12.0 | 2.8 ± 0.7 | 0.211 ± 0.046 |
NA, not available; SE, standard error.
HLA-A genotypes were not available for these CB mononuclear cells. Their flow cytometric phenotypes of HLA-A2 and HLA-A24 are shown.
To elucidate whether human APCs developed in the HLA class I Tg NSG mice, we analyzed the expression of CD33 and other myeloid-associated antigens using flow cytometry. In the NSG-HLA-A2/HHD mice (n = 4) and NSG-HLA-A24/HHD mice (n = 6), we found that CD14+CD33+HLA-DR+ monocytes, CD123highCD11c− plasmacytoid DCs (pDCs), and CD33+CD11c+HLA-DR+ conventional DCs (cDCs) differentiated in the BM and spleen of HLA class I Tg NSG recipients (Figure 1G-H; frequency in human CD45+ cells: BM [n = 8]: monocytes, 3.4% ± 0.8%; pDCs, 2.6% ± 0.7%; and cDCs, 5.2% ± 1.6%; spleen [n = 7]: monocytes, 0.9% ± 0.5%; pDCs, 0.4% ± 0.2%; and cDCs, 1.0% ± 0.4%). BDCA1+ DCs and BDCA3+ DCs were detected within the cDC fraction (Figure 1I; frequency in human CD33+ cells: BM [n = 8]: BDCA1+ DCs, 9.2% ± 4.7%; and BDCA3+ DCs, 1.0% ± 0.3%; spleen [n = 7]: BDCA1+ DCs, 7.6% ± 4.4%; and BDCA3+ DCs, 0.9% ± 0.2%). Consequently, subsets of human CTLs and human myeloid APCs, required for the establishment of acquired immunity after vaccination, were present for the long-term in HLA class I Tg NSG recipients.
WT1 is expressed in the CD34+CD38− LSC fraction of human AML
On the basis of the previous report showing that WT1 is highly expressed in AML stem cells,20 we examined the expression levels of WT1 in the purified AML CD34+CD38− cells and normal BM CD34+CD38− cells using quantitative reverse-transcription PCR. Expression of WT1 in the CD34+CD38− stem cell fraction was higher in AML samples compared with BM samples from healthy donors (supplemental Figure 2A; [relative mRNA expression of WT1 corrected by glyceraldehyde-3-phosphate dehydrogenase] healthy donors, 2.84 ± 2.02 × 10−4 [n = 7]; AML patients, 1.07 ± 0.25 × 10−2 [n = 38]; P = .0002 by 2-tailed Student t test). Western blot analysis confirmed the expression of WT1 in several leukemia cell lines and CD34+CD38− cells from AML patient samples at the protein level (supplemental Figure 2B).
Immunization induced WT1-specific human CTLs in the HLA class I Tg NSG recipients
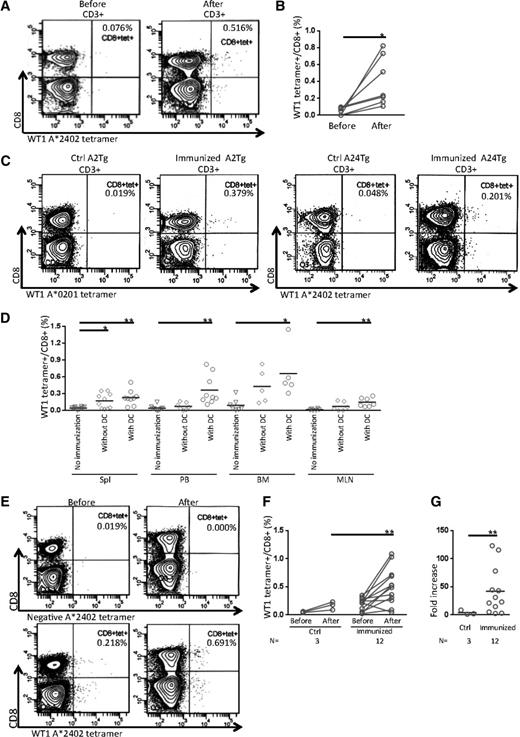
We then performed immunization experiments to evaluate the induction of antigen-specific human CTLs in the HLA class I Tg NSG mice. To do so, we chose WT1 as an antigen and poly I:C as an adjuvant. In addition, we prepared autologous or HLA class I–matched allogeneic WT1 peptide–loaded DCs. In immunized HLA class I Tg recipients (Table 1), we found a significant increase of WT1-specific tetramer-positive (tetramer+) cells in recipients’ PB after immunization (Figure 2A-B; 0.044% ± 0.017% and 0.399% ± 0.110% before and after immunization, respectively [n = 7]; P < .05 by 2-tailed paired Student t test). Compared with unvaccinated mice, the frequencies of WT1 tetramer+ CTLs in spleen were increased significantly in the recipients of the vaccine groups both with and without WT1 peptide–loaded DCs (Figure 2C-D; no immunization control, 0.041% ± 0.007% [n = 10]; immunized without DCs, 0.170% ± 0.043% [n = 10]; P = .0146; immunized with DCs, 0.230% ± 0.044% [n = 9]; P = .0027). In the recipients vaccinated with WT1 peptide–loaded DCs, WT1-specific CTLs were also increased in the PB, BM, and MLN; however, the increases in these organs were not significant in the group immunized without DCs (Figure 2D; WT1 tetramer+/CD8+ T cells (%): PB: no immunization control, 0.038% ± 0.014% [n = 10]; immunized without DCs, 0.072% ± 0.024% [n = 7]; immunized with DCs, 0.361% ± 0.088% [n = 9]; P = .0061; BM: no immunization control, 0.090% ± 0.043% [n = 7]; immunized without DCs, 0.429% ± 0.136% [n = 5]; immunized with DCs, 0.658% ± 0.203% [n = 5]; P = .0473; MLN: no immunization control, 0.013% ± 0.006% [n = 6]; immunized without DCs, 0.071% ± 0.032% [n = 6]; immunized with DCs, 0.146% ± 0.030% [n = 7]; P = .0040).
Immunization of HLA class I Tg NSG recipients using a WT1 antigen. (A) Representative contour plots show development of WT1-specific CD8+ CTLs in PB from an HLA class I Tg NSG recipient before (left) and after (right) immunization using HLA-A*2402 WT1(mutant)235-243 tetramers. (B) Frequencies of WT1-specific tetramer+ CTLs in the PB of HLA class I Tg NSG recipients were increased after immunization (n = 7). (C) Representative flow cytometry plots of tetramer assay in spleens from HLA class I Tg NSG recipients at day of euthanization. Data for the control group and the DC vaccine group in NSG-HLA-A2/HHD recipients (left 2 plots) and NSG-HLA-A24/HHD recipients (right 2 plots) are shown. (D) Summary of tetramer analysis in each organ at day of euthanization. Open inverted triangles, diamonds, and circles indicate the groups of no immunization control, immunized without DCs, and immunized with DCs, respectively. The frequencies of WT1 tetramer+ cells gated on CD8+ T cells in spleen, PB, BM, and MLN are plotted. (E) Representative contour plots of tetramer assay before and after in vitro expansion of CTLs derived from spleens of HLA class I Tg NSG recipients in the group immunized with DCs. Isotype control (upper) and WT1 tetramer (lower). (F) Frequencies of WT1 tetramer+ spleen-derived CTLs (%) before and after in vitro expansion. (G) Fold increase of tetramer+ CTL numbers before and after in vitro expansion. Bars indicate the mean value. *P < .05 and **P < .01 by 2-tailed Student t test. Ctrl, control.
Immunization of HLA class I Tg NSG recipients using a WT1 antigen. (A) Representative contour plots show development of WT1-specific CD8+ CTLs in PB from an HLA class I Tg NSG recipient before (left) and after (right) immunization using HLA-A*2402 WT1(mutant)235-243 tetramers. (B) Frequencies of WT1-specific tetramer+ CTLs in the PB of HLA class I Tg NSG recipients were increased after immunization (n = 7). (C) Representative flow cytometry plots of tetramer assay in spleens from HLA class I Tg NSG recipients at day of euthanization. Data for the control group and the DC vaccine group in NSG-HLA-A2/HHD recipients (left 2 plots) and NSG-HLA-A24/HHD recipients (right 2 plots) are shown. (D) Summary of tetramer analysis in each organ at day of euthanization. Open inverted triangles, diamonds, and circles indicate the groups of no immunization control, immunized without DCs, and immunized with DCs, respectively. The frequencies of WT1 tetramer+ cells gated on CD8+ T cells in spleen, PB, BM, and MLN are plotted. (E) Representative contour plots of tetramer assay before and after in vitro expansion of CTLs derived from spleens of HLA class I Tg NSG recipients in the group immunized with DCs. Isotype control (upper) and WT1 tetramer (lower). (F) Frequencies of WT1 tetramer+ spleen-derived CTLs (%) before and after in vitro expansion. (G) Fold increase of tetramer+ CTL numbers before and after in vitro expansion. Bars indicate the mean value. *P < .05 and **P < .01 by 2-tailed Student t test. Ctrl, control.
To evaluate the antigen-specific proliferation of CTLs, 2 to 10 × 106 splenocytes of recipients were further stimulated with WT1 peptide–pulsed autologous LCLs in vitro. After 4 weeks, the frequency of WT1 tetramer+ CTLs derived from splenocytes was higher in the vaccinated recipients than in unvaccinated mice (Figure 2E-F; WT1 tetramer+/CD8+ (%) before and after in vitro expansion: no immunization group, 0.050% ± 0.006% and 0.168% ± 0.042%, respectively [n = 3]; immunized group, 0.194% ± 0.032% and 0.511% ± 0.095%, respectively [n = 12]; P = .0057). The degree of increase in the number of WT1-specific CTLs after expansion was also higher in the immunized recipients (Figure 2G; fold increase of WT1 tetramer+CD8+ cell counts before and after in vitro expansion: no immunization group, 4.06 ± 2.34 [n = 3]; immunized group, 42.13 ± 12.1 [n = 12]; P = .0097).
Transplant of WT1-TCR-transduced HSCs into NSG-HLA-A24/HHD mice reconstituted WT1-specific CTLs in vivo
As another way of inducing antigen-specific human CTLs in vivo, we evaluated the differentiation of human T cells from antigen-specific TCR-transduced HSCs. We constructed a lentiviral vector encoding WT1-TCR Vα and Vβ genes. We transduced the TCR Vα and Vβ genes into purified human CB HSCs using the lentiviral vector (Figure 3A; supplemental Figure 4A), and purified CD34+GFP+ cells by FACS. Both HLA-matched and -mismatched CBs were used as HSC sources (Table 2). Then we transplanted GFP-labeled gene-transduced CD34+ cells into NSG-HLA-A24/HHD newborn mice. After 4 to 6 months, multilineage human hematopoietic cells developed (Figure 3B-C; supplemental Figure 4B; spleen [n = 7 nontransduced and GFP control recipients]: B cells, 56.9% ± 12.2% and T cells, 34.4% ± 13.1%; [n = 15 WT1-TCR gene–transduced HSC recipients]: B cells, 71.5% ± 4.3% and T cells, 9.8% ± 4.0%; thymus [n = 6 recipients of nontransduced or control vector–transduced HSCs]: B cells, 12.2% ± 4.9% and T cells, 53.1% ± 9.2%; and [n = 15 WT1-TCR gene–transduced HSC recipients]: B cells, 5.2% ± 2.3% and T cells, 28.0% ± 6.0%). CD4+ and CD8+ T cells were identified in the spleen of control NSG recipients and NSG recipients transplanted with WT1-TCR-transduced HSCs. In the thymus, double-positive T cells and single-positive T cells were found in both recipients (Figure 3D). Engraftment levels of human CD45+ cells were not statistically different between control mice and TCR-transduced mice (Table 2).
Transplant of WT1-TCR gene–transduced HSCs into NSG-HLA-A24/HHD recipients. (A) Purification of CB CD34+CD38− cells using a CD34+ cell–enriched CB sample (left). WT1-TCR Vα and Vβ genes were transduced into the isolated cells with a lentiviral vector at a multiplicity of infection of 100. After 5 days of incubation, GFP+ cells were sorted and transplanted into NSG-HLA-A24/HHD newborn mice (right). (B) Multilineage analysis of WT1-TCR gene–transduced NSG-HLA-A24/HHD recipients. Representative plots of spleen (upper) and thymus (lower) are shown. (C) Frequencies of B cells and T cells gated on human CD45+ leukocytes in the spleen and thymus of NSG-HLA-A24/HHD recipients transplanted with nontransduced/GFP control–transduced or WT1-TCR gene–transduced HSCs. (D) Frequencies of human T-cell subsets in the spleen and thymus of NSG-HLA-A24/HHD WT1-TCR gene–transduced HSC recipients (spleen: [n = 6 nontransduced or GFP control recipients] CD4+, 64.3% ± 3.1% and CD8+, 28.5% ± 3.5%; [n = 11 WT1-TCR gene–transduced HSC recipients] CD4+, 50.8% ± 7.4% and CD8+, 29.0% ± 5.3%; thymus: [n = 5 nontransduced or GFP control recipients] CD4+, 46.7% ± 7.1%; CD8+, 29.7% ± 3.8%; and double-positive (DP), 20.4% ± 5.6%; [n = 15 WT1-TCR gene–transduced HSC recipients] CD4+, 25.1% ± 3.8%; CD8+, 35.2% ± 4.3%; and DP, 33.6% ± 6.6%). Open and closed circles indicate nontransduced and GFP control recipients, respectively. Bars indicate the mean value. FSC, forward scatter.
Transplant of WT1-TCR gene–transduced HSCs into NSG-HLA-A24/HHD recipients. (A) Purification of CB CD34+CD38− cells using a CD34+ cell–enriched CB sample (left). WT1-TCR Vα and Vβ genes were transduced into the isolated cells with a lentiviral vector at a multiplicity of infection of 100. After 5 days of incubation, GFP+ cells were sorted and transplanted into NSG-HLA-A24/HHD newborn mice (right). (B) Multilineage analysis of WT1-TCR gene–transduced NSG-HLA-A24/HHD recipients. Representative plots of spleen (upper) and thymus (lower) are shown. (C) Frequencies of B cells and T cells gated on human CD45+ leukocytes in the spleen and thymus of NSG-HLA-A24/HHD recipients transplanted with nontransduced/GFP control–transduced or WT1-TCR gene–transduced HSCs. (D) Frequencies of human T-cell subsets in the spleen and thymus of NSG-HLA-A24/HHD WT1-TCR gene–transduced HSC recipients (spleen: [n = 6 nontransduced or GFP control recipients] CD4+, 64.3% ± 3.1% and CD8+, 28.5% ± 3.5%; [n = 11 WT1-TCR gene–transduced HSC recipients] CD4+, 50.8% ± 7.4% and CD8+, 29.0% ± 5.3%; thymus: [n = 5 nontransduced or GFP control recipients] CD4+, 46.7% ± 7.1%; CD8+, 29.7% ± 3.8%; and double-positive (DP), 20.4% ± 5.6%; [n = 15 WT1-TCR gene–transduced HSC recipients] CD4+, 25.1% ± 3.8%; CD8+, 35.2% ± 4.3%; and DP, 33.6% ± 6.6%). Open and closed circles indicate nontransduced and GFP control recipients, respectively. Bars indicate the mean value. FSC, forward scatter.
NSG-HLA-A24/HHD recipients transplanted with WT1-TCR-transduced HSCs
| Recipient ID . | CB ID . | HLA . | PB chimerism, % . | PB GFP+/hCD45+,% . | Spl chimerism, % . | Spl GFP+/hCD45+, % . | Spl CD3+/hCD45+, % . | Spl WT1 tet+/CD8+, % . |
|---|---|---|---|---|---|---|---|---|
| Nontransduced (n = 4) | ||||||||
| A24-20 | 23-1 | A1101/2601 | 60.0 | — | 84.3 | — | 68.9 | 0.067 |
| A24-21 | 23-44 | A0206/3303 | 87.3 | — | 97.9 | — | 35.3 | 0.021 |
| A24-22 | 23-44 | A0206/3303 | 81.2 | — | 95.3 | — | 92.8 | 0.015 |
| A24-23 | 24-11 | A2402/− | 80.3 | — | 94.3 | — | 26.7 | 0.048 |
| Mean ± SE | 77.2 ± 5.9 | — | 93.0 ± 3.0 | — | 55.9 ± 15.3 | 0.038 ± 0.012 | ||
| GFP control (n = 3) | ||||||||
| A24-GFP-1 | 24-7 | A3101/− | 18.5 | 90.3 | 64.4 | 98.8 | 12.7 | 0.000 |
| A24-GFP-2 | 24-7 | A3101/− | 1.1 | 100.0 | 2.3 | 99.4 | 1.2 | NA |
| A24-GFP-3 | 24-21 | A2402/− | 57.7 | 14.0 | 87.1 | 22.4 | 46.7 | 0.013 |
| Mean ± SE | 25.8 ± 16.7 | 68.1 ± 27.2 | 51.3 ± 25.4 | 73.6 ± 25.6 | 20.2 ± 13.6 | 0.006 ± 0.006 | ||
| WT1-TCR (n = 15) | ||||||||
| A24-TCR-1 | 23-1 | A1101/2601 | 47.0 | 7.5 | 80.9 | 31.6 | 50.2 | 0.455 |
| A24-TCR-2 | 23-1 | A1101/2601 | 63.9 | 25.7 | 90.2 | 33.8 | 78.6 | 0.186 |
| A24-TCR-3 | 23-44 | A0206/3303 | 45.3 | 21.3 | 79.9 | 32.1 | 25.4 | 0.216 |
| A24-TCR-4 | 23-44 | A0206/3303 | 51.5 | 20.2 | 67.4 | 27.8 | 10.9 | 0.165 |
| A24-TCR-5 | 23-44 | A0206/3303 | 53.0 | 24.7 | 86.8 | 36.3 | 18.4 | 0.118 |
| A24-TCR-6 | 23-44 | A0206/3303 | 27.3 | 27.2 | 75.9 | 73.2 | 0.7 | NA |
| A24-TCR-7 | 24-7 | A3101/− | 9.1 | 42.7 | 48.0 | 83.2 | 0.3 | NA |
| A24-TCR-8 | 24-7 | A3101/− | 0.6 | 62.2 | 11.0 | 79.0 | 3.3 | NA |
| A24-TCR-9 | 24-13 | A2402/− | 78.5 | 62.8 | 89.4 | 74.5 | 9.9 | 0.636 |
| A24-TCR-10 | 24-15 | A3101/3303 | 50.7 | 94.6 | 76.4 | 92.8 | 1.4 | 0.585 |
| A24-TCR-11 | 24-21 | A2402/− | 51.5 | 2.0 | 87.2 | 7.2 | 68.4 | 0.073 |
| A24-TCR-12 | 24-16 | A0201/3303 | 11.9 | 92.1 | 34.0 | 95.8 | 13.5 | NA |
| A24-TCR-13 | 24-16 | A0201/3303 | 28.3 | 18.1 | 74.2 | 64.5 | 46.7 | 0.410 |
| A24-TCR-14 | 24-15 | A3101/3303 | 10.1 | 99.0 | 54.6 | 98.7 | 2.3 | NA |
| A24-TCR-15 | 24-40 | A2402/− | 16.2 | 90.2 | 58.6 | 95.7 | 1.0 | NA |
| Mean ± SE | 36.3 ± 6.2 | 46.0 ± 9.2 | 67.6 ± 6.1 | 61.8 ± 8.2 | 22.1 ± 7.1 | 0.316 ± 0.070 |
| Recipient ID . | CB ID . | HLA . | PB chimerism, % . | PB GFP+/hCD45+,% . | Spl chimerism, % . | Spl GFP+/hCD45+, % . | Spl CD3+/hCD45+, % . | Spl WT1 tet+/CD8+, % . |
|---|---|---|---|---|---|---|---|---|
| Nontransduced (n = 4) | ||||||||
| A24-20 | 23-1 | A1101/2601 | 60.0 | — | 84.3 | — | 68.9 | 0.067 |
| A24-21 | 23-44 | A0206/3303 | 87.3 | — | 97.9 | — | 35.3 | 0.021 |
| A24-22 | 23-44 | A0206/3303 | 81.2 | — | 95.3 | — | 92.8 | 0.015 |
| A24-23 | 24-11 | A2402/− | 80.3 | — | 94.3 | — | 26.7 | 0.048 |
| Mean ± SE | 77.2 ± 5.9 | — | 93.0 ± 3.0 | — | 55.9 ± 15.3 | 0.038 ± 0.012 | ||
| GFP control (n = 3) | ||||||||
| A24-GFP-1 | 24-7 | A3101/− | 18.5 | 90.3 | 64.4 | 98.8 | 12.7 | 0.000 |
| A24-GFP-2 | 24-7 | A3101/− | 1.1 | 100.0 | 2.3 | 99.4 | 1.2 | NA |
| A24-GFP-3 | 24-21 | A2402/− | 57.7 | 14.0 | 87.1 | 22.4 | 46.7 | 0.013 |
| Mean ± SE | 25.8 ± 16.7 | 68.1 ± 27.2 | 51.3 ± 25.4 | 73.6 ± 25.6 | 20.2 ± 13.6 | 0.006 ± 0.006 | ||
| WT1-TCR (n = 15) | ||||||||
| A24-TCR-1 | 23-1 | A1101/2601 | 47.0 | 7.5 | 80.9 | 31.6 | 50.2 | 0.455 |
| A24-TCR-2 | 23-1 | A1101/2601 | 63.9 | 25.7 | 90.2 | 33.8 | 78.6 | 0.186 |
| A24-TCR-3 | 23-44 | A0206/3303 | 45.3 | 21.3 | 79.9 | 32.1 | 25.4 | 0.216 |
| A24-TCR-4 | 23-44 | A0206/3303 | 51.5 | 20.2 | 67.4 | 27.8 | 10.9 | 0.165 |
| A24-TCR-5 | 23-44 | A0206/3303 | 53.0 | 24.7 | 86.8 | 36.3 | 18.4 | 0.118 |
| A24-TCR-6 | 23-44 | A0206/3303 | 27.3 | 27.2 | 75.9 | 73.2 | 0.7 | NA |
| A24-TCR-7 | 24-7 | A3101/− | 9.1 | 42.7 | 48.0 | 83.2 | 0.3 | NA |
| A24-TCR-8 | 24-7 | A3101/− | 0.6 | 62.2 | 11.0 | 79.0 | 3.3 | NA |
| A24-TCR-9 | 24-13 | A2402/− | 78.5 | 62.8 | 89.4 | 74.5 | 9.9 | 0.636 |
| A24-TCR-10 | 24-15 | A3101/3303 | 50.7 | 94.6 | 76.4 | 92.8 | 1.4 | 0.585 |
| A24-TCR-11 | 24-21 | A2402/− | 51.5 | 2.0 | 87.2 | 7.2 | 68.4 | 0.073 |
| A24-TCR-12 | 24-16 | A0201/3303 | 11.9 | 92.1 | 34.0 | 95.8 | 13.5 | NA |
| A24-TCR-13 | 24-16 | A0201/3303 | 28.3 | 18.1 | 74.2 | 64.5 | 46.7 | 0.410 |
| A24-TCR-14 | 24-15 | A3101/3303 | 10.1 | 99.0 | 54.6 | 98.7 | 2.3 | NA |
| A24-TCR-15 | 24-40 | A2402/− | 16.2 | 90.2 | 58.6 | 95.7 | 1.0 | NA |
| Mean ± SE | 36.3 ± 6.2 | 46.0 ± 9.2 | 67.6 ± 6.1 | 61.8 ± 8.2 | 22.1 ± 7.1 | 0.316 ± 0.070 |
Abbreviations are explained in Table 1.
–, no GFP+ cells in the nontransduced group.
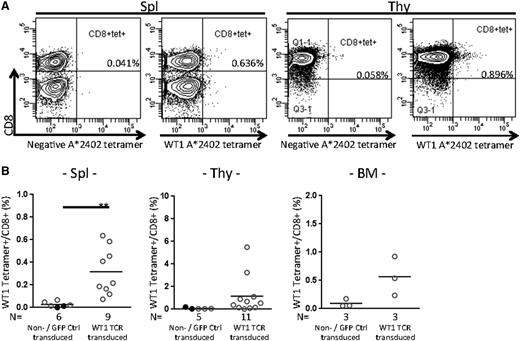
At the day of euthanization, WT1 tetramer+CD8+ cells were present at frequencies of 0.316% ± 0.070% (n = 9), 1.158% ± 0.513% (n = 11), and 0.565% ± 0.200% (n = 3) in the spleen, thymus, and BM of WT1-TCR-transduced HSC recipients, respectively (Figure 4).
CTL analysis of NSG-HLA-A24/HHD WT1-TCR gene–transduced HSC recipients. (A) Representative flow cytometry plots showing tetramer analysis in the spleen (left 2 plots) and thymus (right 2 plots) of recipients transplanted with WT1-TCR gene–transduced HSCs. (B) The frequencies of WT1-specific CTLs in spleen were significantly increased in the recipients of WT1-TCR gene–transduced HSCs compared with those of nontransduced or control vector–transduced HSCs. The frequencies of WT1 tetramer+ gated on CD8+ T cells (%) are shown for spleen (left), thymus (center), and BM (right). Spleen: n = 6 nontransduced or GFP control vector–transduced HSC recipients, 0.027% ± 0.010%; n = 9 WT1-TCR gene–transduced HSC recipients, 0.316% ± 0.070%, **P = .0055; thymus: n = 5 nontransduced or GFP control vector–transduced HSC recipients, 0.074% ± 0.031%; n = 11 WT1-TCR gene–transduced HSC recipients, 1.158% ± 0.513%; and BM: n = 3 nontransduced or GFP control vector–transduced HSC recipients, 0.093% ± 0.041%; n = 3 WT1-TCR gene–transduced HSC recipients, 0.565% ± 0.200%. tet, tetramer.
CTL analysis of NSG-HLA-A24/HHD WT1-TCR gene–transduced HSC recipients. (A) Representative flow cytometry plots showing tetramer analysis in the spleen (left 2 plots) and thymus (right 2 plots) of recipients transplanted with WT1-TCR gene–transduced HSCs. (B) The frequencies of WT1-specific CTLs in spleen were significantly increased in the recipients of WT1-TCR gene–transduced HSCs compared with those of nontransduced or control vector–transduced HSCs. The frequencies of WT1 tetramer+ gated on CD8+ T cells (%) are shown for spleen (left), thymus (center), and BM (right). Spleen: n = 6 nontransduced or GFP control vector–transduced HSC recipients, 0.027% ± 0.010%; n = 9 WT1-TCR gene–transduced HSC recipients, 0.316% ± 0.070%, **P = .0055; thymus: n = 5 nontransduced or GFP control vector–transduced HSC recipients, 0.074% ± 0.031%; n = 11 WT1-TCR gene–transduced HSC recipients, 1.158% ± 0.513%; and BM: n = 3 nontransduced or GFP control vector–transduced HSC recipients, 0.093% ± 0.041%; n = 3 WT1-TCR gene–transduced HSC recipients, 0.565% ± 0.200%. tet, tetramer.
Human CTLs developed in the NSG-HLA-A24/HHD mice were stimulated in vitro using HLA-A*2402(+) WT1 peptide–pulsed LCLs for further expansion (supplemental Figure 5). When HLA-A*2402-mismatched CB HSCs were transplanted into NSG-HLA-A24/HHD newborn mice, WT1 peptide–pulsed, HLA-A*2402-matched allogeneic CB LCLs were used as stimulators to expand WT1-specific CTLs in vitro. After 2 to 3 weeks, the frequency of WT1 tetramer+ CTLs derived from splenocytes of the recipients was significantly increased (Figure 5A-B; WT1 tetramer+/CD8+ T cells (%): BM [n = 5]: before, 0.626% ± 0.140% and after, 11.57% ± 10.23%; P = .3880; spleen [n = 9]: before, 0.316% ± 0.070% and after, 10.12% ± 7.781%; P = .0197; thymus [n = 4]: before, 1.533% ± 1.282% and after, 23.95% ± 14.10%; P = .0561). The cell numbers of WT1 tetramer+ CTLs derived from BM, spleen, and thymus were significantly increased after in vitro expansion (Figure 5C; cell numbers of WT1 tetramer+CD8+ T cells: BM [n = 5]: before, 8.1 × 101 ± 4.5 × 101 and after, 1.5 × 106 ± 1.5 × 106; *P = .0253; spleen [n = 9]: before, 2.7 × 102 ± 1.1 × 102 and after, 9.4 × 105 ± 8.9 × 105; P = .0013; thymus [n = 4]: before, 4.0 × 102 ± 2.4 × 102 and after, 1.9 × 106 ± 1.8 × 106; P = .0102).
In vitro expanded CTLs derived from NSG-HLA-A24/HHD WT1-TCR gene–transduced HSC recipients exerted HLA-restricted antigen-specific response. (A) Representative flow cytometry plots showing WT1/HLA-A*2402 tetramer analysis before and after in vitro stimulation of CTLs from BM (top), spleen (middle), and thymus (bottom) of NSG-HLA-A24/HHD recipients transplanted with WT1-TCR–transduced HSCs. The frequencies (B) and cell numbers (C) of WT1-specific tetramer+ CTLs derived from WT1-TCR-transduced HSC recipients before and 1 week after the second in vitro stimulation. *P < .05 and **P < .01 by ratio Student t test. (D) After expansion, recipient-derived WT1-specific CTLs exerted IFN-γ production in response to WT1 peptide–pulsed LCLs (top row), but were not responsive against peptide-unpulsed LCLs (middle row). The addition of anti-HLA class I antibody reduced the WT1 peptide–specific cytokine production (bottom row). (E) Spot counts of IFN-γ-producing cells (spot-forming cells [SFC]) out of 1 × 104 CTLs after expansion are shown. *P < .05 and **P < .01 by 2-tailed Student t test. (F) Results of 51Cr release assay showing antigen-specific, HLA-restricted cytotoxicity by amplified CTLs derived from WT1-TCR-transduced NSG-HLA-A24/HHD–recipient BM (A24-TCR-2) at indicated effector-to-target cell (E:T) ratios (upper). 51Cr release assays at an E:T ratio of 5:1 in the presence or absence of anti-HLA class I mAb or anti-HLA class II mAb (lower). (G) Cytotoxic activity of WT1-specific CTLs from A24-TCR-2 BM against leukemia cell lines (left). The cytotoxicity of these CTLs was inhibited by adding anti-HLA class I mAb, but not by adding anti-HLA class II mAb (right).
In vitro expanded CTLs derived from NSG-HLA-A24/HHD WT1-TCR gene–transduced HSC recipients exerted HLA-restricted antigen-specific response. (A) Representative flow cytometry plots showing WT1/HLA-A*2402 tetramer analysis before and after in vitro stimulation of CTLs from BM (top), spleen (middle), and thymus (bottom) of NSG-HLA-A24/HHD recipients transplanted with WT1-TCR–transduced HSCs. The frequencies (B) and cell numbers (C) of WT1-specific tetramer+ CTLs derived from WT1-TCR-transduced HSC recipients before and 1 week after the second in vitro stimulation. *P < .05 and **P < .01 by ratio Student t test. (D) After expansion, recipient-derived WT1-specific CTLs exerted IFN-γ production in response to WT1 peptide–pulsed LCLs (top row), but were not responsive against peptide-unpulsed LCLs (middle row). The addition of anti-HLA class I antibody reduced the WT1 peptide–specific cytokine production (bottom row). (E) Spot counts of IFN-γ-producing cells (spot-forming cells [SFC]) out of 1 × 104 CTLs after expansion are shown. *P < .05 and **P < .01 by 2-tailed Student t test. (F) Results of 51Cr release assay showing antigen-specific, HLA-restricted cytotoxicity by amplified CTLs derived from WT1-TCR-transduced NSG-HLA-A24/HHD–recipient BM (A24-TCR-2) at indicated effector-to-target cell (E:T) ratios (upper). 51Cr release assays at an E:T ratio of 5:1 in the presence or absence of anti-HLA class I mAb or anti-HLA class II mAb (lower). (G) Cytotoxic activity of WT1-specific CTLs from A24-TCR-2 BM against leukemia cell lines (left). The cytotoxicity of these CTLs was inhibited by adding anti-HLA class I mAb, but not by adding anti-HLA class II mAb (right).
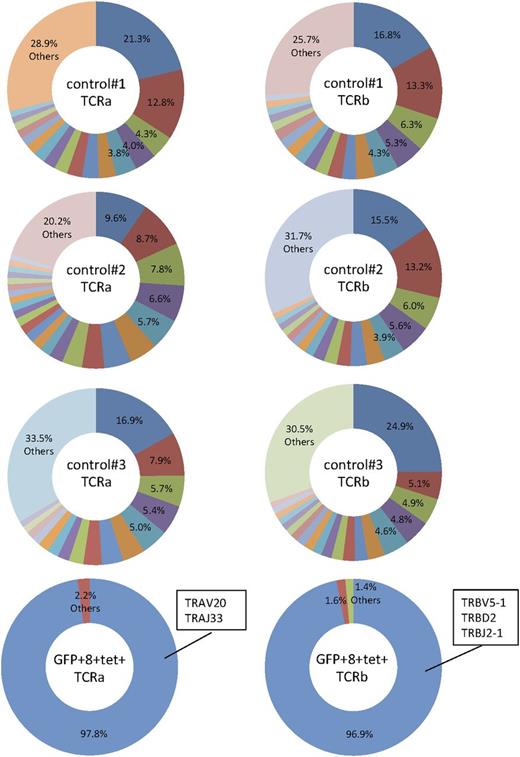
To elucidate whether the GFP+ WT1 tetramer+CD8+ T cells retain specific TCRs, we performed a repertoire analysis of TCRs using MiSeq. The result of repertoire sequences demonstrated that FACS-purified GFP+ WT1 tetramer+CD8+ T cells displayed skewing of both Vα and Vβ repertoires and that the majority of Vα and Vβ chains of the T cells were the transduced Vα and Vβ chains (Figure 6). After the expansion of WT1-specific CTLs for 4 to 5 weeks, we found that the majority of GFP+CD8+ T cells were CD45RA(+)CCR7(−) effector T cells (supplemental Figure 6).
Skewed TCR repertoire in human CD8+ T cells developed in NSG-HLA-A24/HHD WT1-TCR gene–transduced HSC recipients. CD8+GFP+tetramer+ T cells were purified from an NSG-HLA-A24/HHD mouse transplanted with WT1-TCR-transduced human HSCs. Human CD8+ T cells were also purified from control mice engrafted with nontransduced human HSCs. Pie charts depict the major repertoires >1%. The minor repertoires <1% are indicated as “Others.”
Skewed TCR repertoire in human CD8+ T cells developed in NSG-HLA-A24/HHD WT1-TCR gene–transduced HSC recipients. CD8+GFP+tetramer+ T cells were purified from an NSG-HLA-A24/HHD mouse transplanted with WT1-TCR-transduced human HSCs. Human CD8+ T cells were also purified from control mice engrafted with nontransduced human HSCs. Pie charts depict the major repertoires >1%. The minor repertoires <1% are indicated as “Others.”
We also evaluated the antigen-specific cytokine production by the CTLs with the ELISpot assay. Recipient-derived WT1-specific CTLs produced IFN-γ against WT1 peptide–pulsed, but not peptide-unpulsed, HLA-A*2402(+) LCLs. The addition of anti-HLA class I antibody significantly reduced the number of IFN-γ-producing cells against WT1 peptide–pulsed LCLs (Figure 5D-E). WT1-specific CTLs also produced tumor necrosis factor α (supplemental Figure 7).
Using 51Cr release assay, we evaluated cytotoxicity by the amplified CTLs derived from a WT1-TCR-transduced NSG-HLA-A24/HHD recipient BM (A24-TCR-2) (Figure 5F-G). Because this donor CB sample used for the transplant of WT1-TCR-transduced HSC was HLA-A*2402 mismatched (Table 2; CB23-1), allogeneic HLA-A*2402-matched LCLs were used for in vitro stimulation. Cytotoxic activities were observed against allogeneic WT1 peptide–pulsed HLA-A*2402-matched LCLs (Figure 5F; LCL#1, derived from CB23-3), but not against peptide-unpulsed LCL#1 and autologous HLA-A*2402-mismachted LCLs (Figure 5F, upper; LCL#2, derived from CB23-1) with or without peptide loading. WT1(+) HLA-A*2402(+) KAZZ leukemia cell lines were lysed by these CTLs. Although HLA-A*2402(−) K562 leukemia cell lines were not lysed, HLA-A*2402 gene–transduced K562 cells became susceptible to lysis by these CTLs (Figure 5G, left). The cytotoxic activities of CTLs against WT1 peptide–pulsed LCL#1 and HLA-A*2402-expressing LCLs were inhibited by anti-HLA class I mAb, but not by anti-HLA class II mAb (Figure 5F, lower, and G, right). We further aimed to determine the effect of WT1-specific CTLs on the in vivo growth of leukemic cells. To this end, we used as effector cells WT1-specific CTLs obtained from spleens of NSG-HLA-A24/HHD recipients engrafted with human HSCs transduced with WT1-TCR genes. HLA-A24-expressing K562 cells were used as target cells. We cultured the target K562 cells with WT1-specific CTLs ex vivo, and then inoculated the cultured K562 cells subcutaneously into NSG mice. At 3 to 7 days after 3 weekly transfers of WT1-specific CTLs, we found inhibition of subcutaneous tumor formation (Table 3; supplemental Figure 8). These results indicate that WT1-TCR-transduced human HSCs can induce specific and functional CTLs and multilineage immune subsets in HLA class I Tg NSG recipients.
NSG recipients transplanted with HLA-A24-expressing K562 cells
| Mouse . | Day . | Coculture . | IV transfer of CTLs . | Size of tumor, g . | ||||
|---|---|---|---|---|---|---|---|---|
| No. of K562 cells . | No. of CTLs . | No. of cells . | No. of cells . | No. of cells . | With CTLs . | Without CTLs . | ||
| 1 | 24 | 1.0e+06 | 5.0e+06 | 3.4e+06 | 5.0e+06 | 6.0e+06 | 1.69 | 3.61 |
| 2 | 24 | 1.0e+06 | 5.0e+06 | 3.0e+06 | 4.0e+06 | 2.0e+06 | 2.49 | 5.3 |
| 3 | 25 | 4.0e+05 | 4.0e+06 | 2.0e+06 | 1.3e+06 | 8.0e+05 | 0.592 | 0.615 |
| 4 | 24 | 7.5e+05 | 5.7e+06 | 2.5e+06 | 1.2e+06 | 3.1e+06 | 0.697 | 1.652 |
| 5 | 28 | 4.8e+05 | 2.4e+06 | 3.0e+06 | 5.0e+06 | 5.4e+06 | 0.107 | 0.283 |
| Mouse . | Day . | Coculture . | IV transfer of CTLs . | Size of tumor, g . | ||||
|---|---|---|---|---|---|---|---|---|
| No. of K562 cells . | No. of CTLs . | No. of cells . | No. of cells . | No. of cells . | With CTLs . | Without CTLs . | ||
| 1 | 24 | 1.0e+06 | 5.0e+06 | 3.4e+06 | 5.0e+06 | 6.0e+06 | 1.69 | 3.61 |
| 2 | 24 | 1.0e+06 | 5.0e+06 | 3.0e+06 | 4.0e+06 | 2.0e+06 | 2.49 | 5.3 |
| 3 | 25 | 4.0e+05 | 4.0e+06 | 2.0e+06 | 1.3e+06 | 8.0e+05 | 0.592 | 0.615 |
| 4 | 24 | 7.5e+05 | 5.7e+06 | 2.5e+06 | 1.2e+06 | 3.1e+06 | 0.697 | 1.652 |
| 5 | 28 | 4.8e+05 | 2.4e+06 | 3.0e+06 | 5.0e+06 | 5.4e+06 | 0.107 | 0.283 |
Discussion
Evoking immune responses against specific antigens requires HLA-restricted interaction between APCs and effector cells. In this study, we examined whether active immunization with vaccines can prime HLA-restricted antigen-specific human CTLs in mice expressing HLA class I molecules HLA-A*0201 and HLA-A*2402, the most common alleles in Caucasian26 and Japanese populations, respectively.27 First, we confirmed HLA class I expression in murine thymic epithelial cells from the previously reported NSG-HLA-A2/HHD24,28 and from the newly developed NSG-HLA-A24/HHD strains. In HLA class I Tg NSG mice transplanted with HLA class I–matched CB HSCs, human memory T cells expressing cytotoxic molecules and human HLA-DR-positive antigen-presenting myeloid cells, including BDCA1+ or BDCA3+ cDCs, were detected in the recipient BM and spleen. After vaccination using adjuvant mixed 9-mer WT1 peptides, both with and without the support of ex vivo prepared antigen-loaded autologous DCs, antigen-specific CTLs increased in vivo. The frequencies of WT1-specific tetramer+CD8+ T cells in the vaccinated recipients were comparable to those in the AML patients who received vaccination using WT1 antigen.16,17,22,23 When we compared the peptide-vaccination and DC-vaccination groups, we found more efficient induction of WT1-specific CD8+ T cells with the support of autologous DCs, which might be accounted for by the incomplete DC development in the lymph nodes of HSC-engrafted NSG mice.
Toll-like receptor 3 (TLR3)-expressing human BDCA3+ cDCs are regarded as a functional equivalent of murine CD8a+ DCs, which play crucial role in the induction of CTL responses against cancer cells and viruses.29-32 The TLR3 agonist poly I:C has been shown to function as an adjuvant, and induces cellular immune responses in a mouse model33 and in clinical trials.34 Because we confirmed the expression of TLR3 in DCs in human HSC-engrafted NSG mice, we chose poly I:C as an adjuvant. The frequency of BDCA3+ DCs was increased after vaccination of poly I:C mixed with WT1 peptides (supplemental Figure 3).
Although adoptive immunotherapy with peripheral T cells that express antigen-specific TCR genes has been evaluated in clinical trials,9 there are potential problems such as limited ex vivo expansion of peripheral T cells35 and a risk of generating autoreactive T-cell clones due to mispairing between Tg and endogenous TCR chains.36 Recently, using a retroviral vector encoding small interfering RNAs targeting endogenous TCR genes,37 we transduced WT1-TCR genes into peripheral T cells and demonstrated their in vivo cytotoxicity against leukemia cells.38
In the current study, we aimed to transfer WT1-TCR genes into HSCs to generate Tg T cells in vivo for long-term function. It was reasoned that these T cells would undergo negative selection resulting in the elimination of autoreactive T cells.39 During a median follow-up of 26 weeks, the recipients of WT1-TCR gene–transduced HSCs did not show any signs of severe graft-versus-host-disease such as weight loss, skin inflammation, and diarrhea. The recipient-derived WT1-specific CTLs retained the capacity to proliferate in vitro and exerted antigen-specific cytokine response and lytic activity, indicating that the HSCs generated functional Tg CTLs in vivo.
Our results supported previous reports describing the development of Tg CTLs specific for MART-1 (melanoma antigen recognized by T cells).40-42 Tg MART-1-specific CD8+ T cells were detected in BM/liver/thymus humanized mice43 transplanted with TCR gene–transduced fetal liver–derived CD34+ HSPCs40 or in HLA-A*0201 Tg NSG mice transplanted with TCR-transduced CB CD34+ HSPCs.42 Through the TCR Vβ repertoire analysis of MART-1-specific tetramer+ T cells in the recipients, both groups demonstrated that the allelic exclusion by exogenous TCRs suppressed the rearrangement of endogenous human TCR genes during thymocyte differentiation and reduced the risk of generating autoreactive CTL clones.
The frequency of WT1-specific Tg CTLs in our recipients was relatively lower compared with that of MART-1-specific CTLs detected in those reports.40-42 Several differences in experimental settings might explain this disparity. First, we isolated CD34+CD38− CB HSCs for lentiviral TCR transfer, and transduction marker–positive (GFP+) cells for transplant, to exclude the possibility of residual preformed human T cells in the graft. Purified WT1-TCR-transferred HSCs still retained the multilineage differentiation capacity. Second, the variances of gene expression patterns between WT1 and MART-1 might affect their immunogenicity. WT1 is expressed in normal tissues, including podocytes of normal kidney,44 Sertoli cells of testis, and granulosa cells of ovary,45 whereas the expression of MART-1 is localized to melanocytes of skin and retina.46,47
Without expression of human HLA class II molecules, appropriate CD4 T-cell help might be lacking in our recipients, causing a failure of T-cell memory formation, as well as incomplete priming of CD8+ cells in vivo.48 In addition, current xenograft recipient lymph nodes do not fully recapitulate human lymph node architecture (supplemental Figure 9). In the future, we aim to humanize the lymph node microenvironment in the recipient and support the development of lymph node architecture through Tg expression of human cytokines and a more physiological repopulation of human immune cells.
As previously reported,20 we confirmed high expression of WT1 in the CD34+CD38− LSC fraction from patients with AML at mRNA and protein levels. As a marker of minimal residual disease, WT1 mRNA expression in patients with AML has been assessed for the prediction of relapse after chemotherapy49 and HSC transplant.50 Our results might provide some motivation to perform immunotherapeutic targeting of WT1 against persistent LSCs in hematologic remission. Regardless of the HLA-A locus genotype of the donor, we detected functional WT1-specific CTLs in the BM51,52 and spleen of the recipients transplanted with TCR-transduced CB HSCs, suggesting the application for a wide variety of donor-graft combinations in the clinical setting of HSC transplant.
In the current study, we were able to evaluate 2 different options of human immunotherapy in a mouse model. On the basis of these results, the HLA class I Tg NSG xenograft system might serve as a preclinical tool for immunotherapy against human malignancies.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank David Serreze for providing NOD/Lt mice transgenically expressing HLA-A24, and Kiyotaka Kuzushima for providing HLA-A24-expressing K562 cells.
This study was supported by the Basic Science and Platform Technology Program for Innovative Biological Medicine; the Project for Development of Innovative Research on Cancer Therapeutics from Ministry of Education, Culture, Sports, Science, and Technology; The Japan Agency for Medical Research and Development; The Maine Cancer Foundation; and National Institutes of Health National Cancer Institute grants CA034196 and CA171983 and Office of the Director grant R24OD018259.
Authorship
Contribution: Y.N. designed and performed the experiments, analyzed the data, and wrote the manuscript; M.T.-M. performed the sorting experiments and T-cell functional assays; T.O. and H.F. performed the 51Cr releasing assay and analyzed the data; R.O. analyzed the data; T.W. analyzed the genomic data for T cells; N.S. performed the sorting experiments and analyzed the myeloid differentiation; and Y.S., O.O., L.D.S., M.Y., and F.I. designed the research, analyzed the data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Fumihiko Ishikawa, Laboratory for Human Disease Models, RIKEN Center for Integrative Medical Sciences, 1-7-22 Suehiro-cho Tsurumi-ku, Yokohama 230-0045, Japan; e-mail: fumihiko.ishikawa@riken.jp.

![Figure 1. HLA expression and multilineage human immune subsets in HLA class I Tg NSG humanized mice. (A) Reverse-transcription PCR for HLA-A*0201, HLA-A*2402, human β-2-microglobulin (hu-β2M), and murine β-2-microglobulin (ms-β2M) using complementary DNA from BM, spleen, and msCD45−EpCAM+ thymic epithelial cells (TEC) derived from nonengrafted mice. “A2Tg” (left) and “A24Tg” (right) indicate NSG-HLA-A2/HHD and NSG-HLA-A24/HHD, respectively. (B) Representative contour plots indicating the engraftment of hCD45+ leukocytes, hCD3+ T cells, and hCD19+ B cells in the spleen of an NSG-HLA-A24/HHD recipient at 18 weeks after transplant. (C) Engraftment levels of hCD45+ cells in each organ (n = 11 for PB, spleen, BM, MLN, and thymus; n = 10 for axillary lymph nodes [AxLN]). (D) In the CD8+ cell fraction of the spleen of HLA class I Tg NSG recipients, naïve, CM, EM, and EMRA T-cell subsets were identified. (E) The frequencies of CD8+ memory T-cell subsets in the spleen of HLA class I Tg NSG recipients (n = 10). (F) Cytoplasmic expression of granzyme A in CD8+ T-cell subsets from naïve to E phenotype in the spleen of HLA class I Tg NSG recipients (n = 4). (G) Representative contour plots of human myeloid subsets in the BM of an HLA class I Tg NSG recipient. (H) The frequencies of monocytes (Mono; CD14+CD33+HLA-DR+), pDCs (CD123+CD11c−), and cDCs (CD33+HLA-DR+CD11c+) among hCD45+ cells in BM (left) and spleen (right). (I) cDCs were further divided into BDCA1+ and BDCA3+ DCs in BM (n = 8) (left) and spleen (n = 7) (right). Bars indicate the mean value. E, effector; ms, mouse; Spl, spleen; Thy, thymus.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/127/6/10.1182_blood-2014-10-604777/4/m_722f1.jpeg?Expires=1769217408&Signature=LaWezUg5byiGFUmGJ3jv5TmL0fqs5vSRTt2ouXqKbktfZaCRsVs6agpLRXQ3IzxaqKLpU8GKvzzfLMvB1LZSWO87e0fAJ-rVDkyDZ6j02~Z~TMDTX7QExb5Ok3o5x4~YPY64YdLFPFsDWXDzWuWBvPY9nFD6pAnZnmqsStOlnVqJkYucuLvSh6e64pE~q-JgwrNrtk2IJOctNReJROCGEOLQorRtm55qLVeKeoFkak80BKtXLteSICo1VmLFAysSkDeDHph792RazilIne53ozEBKFk5jr5Ed36Fo74hHinAqjoE7W~QDpflY~YeEiLJAcqIGmuU-RLZSGw46m6IhA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 3. Transplant of WT1-TCR gene–transduced HSCs into NSG-HLA-A24/HHD recipients. (A) Purification of CB CD34+CD38− cells using a CD34+ cell–enriched CB sample (left). WT1-TCR Vα and Vβ genes were transduced into the isolated cells with a lentiviral vector at a multiplicity of infection of 100. After 5 days of incubation, GFP+ cells were sorted and transplanted into NSG-HLA-A24/HHD newborn mice (right). (B) Multilineage analysis of WT1-TCR gene–transduced NSG-HLA-A24/HHD recipients. Representative plots of spleen (upper) and thymus (lower) are shown. (C) Frequencies of B cells and T cells gated on human CD45+ leukocytes in the spleen and thymus of NSG-HLA-A24/HHD recipients transplanted with nontransduced/GFP control–transduced or WT1-TCR gene–transduced HSCs. (D) Frequencies of human T-cell subsets in the spleen and thymus of NSG-HLA-A24/HHD WT1-TCR gene–transduced HSC recipients (spleen: [n = 6 nontransduced or GFP control recipients] CD4+, 64.3% ± 3.1% and CD8+, 28.5% ± 3.5%; [n = 11 WT1-TCR gene–transduced HSC recipients] CD4+, 50.8% ± 7.4% and CD8+, 29.0% ± 5.3%; thymus: [n = 5 nontransduced or GFP control recipients] CD4+, 46.7% ± 7.1%; CD8+, 29.7% ± 3.8%; and double-positive (DP), 20.4% ± 5.6%; [n = 15 WT1-TCR gene–transduced HSC recipients] CD4+, 25.1% ± 3.8%; CD8+, 35.2% ± 4.3%; and DP, 33.6% ± 6.6%). Open and closed circles indicate nontransduced and GFP control recipients, respectively. Bars indicate the mean value. FSC, forward scatter.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/127/6/10.1182_blood-2014-10-604777/4/m_722f3.jpeg?Expires=1769217408&Signature=xc84LUOaY9Z8WSa~PzMOHDRXp6BCLR78MkMptgHQ7mN2bblqEq1YKKjrkKOyQvNRblTd2IwPwWtJ6RKx3Av2fFn3jqg6R10U59pVYXa~U9SQCfGTTcp4rtuujg0stF32BkIPjreLDFUL4fhBT9zRWt6249BdX36zo0KHv5eYsJk-~nbAi8uyEZ73Uw-qolWCB3u6SY--fO0wiGjPM446PeQLqvmJmPm~Pch6-5YXPj0unNJ1bs~Q3dzKbflqIT5CoRuTIomc8ZheCAZH10u2xxa5n6hkM-3EOasvGWfq7ysbxzWoMbDihceMgDUFBIbFnHlL6u8FadSgtv6OgSZc6g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 5. In vitro expanded CTLs derived from NSG-HLA-A24/HHD WT1-TCR gene–transduced HSC recipients exerted HLA-restricted antigen-specific response. (A) Representative flow cytometry plots showing WT1/HLA-A*2402 tetramer analysis before and after in vitro stimulation of CTLs from BM (top), spleen (middle), and thymus (bottom) of NSG-HLA-A24/HHD recipients transplanted with WT1-TCR–transduced HSCs. The frequencies (B) and cell numbers (C) of WT1-specific tetramer+ CTLs derived from WT1-TCR-transduced HSC recipients before and 1 week after the second in vitro stimulation. *P < .05 and **P < .01 by ratio Student t test. (D) After expansion, recipient-derived WT1-specific CTLs exerted IFN-γ production in response to WT1 peptide–pulsed LCLs (top row), but were not responsive against peptide-unpulsed LCLs (middle row). The addition of anti-HLA class I antibody reduced the WT1 peptide–specific cytokine production (bottom row). (E) Spot counts of IFN-γ-producing cells (spot-forming cells [SFC]) out of 1 × 104 CTLs after expansion are shown. *P < .05 and **P < .01 by 2-tailed Student t test. (F) Results of 51Cr release assay showing antigen-specific, HLA-restricted cytotoxicity by amplified CTLs derived from WT1-TCR-transduced NSG-HLA-A24/HHD–recipient BM (A24-TCR-2) at indicated effector-to-target cell (E:T) ratios (upper). 51Cr release assays at an E:T ratio of 5:1 in the presence or absence of anti-HLA class I mAb or anti-HLA class II mAb (lower). (G) Cytotoxic activity of WT1-specific CTLs from A24-TCR-2 BM against leukemia cell lines (left). The cytotoxicity of these CTLs was inhibited by adding anti-HLA class I mAb, but not by adding anti-HLA class II mAb (right).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/127/6/10.1182_blood-2014-10-604777/4/m_722f5.jpeg?Expires=1769217408&Signature=vv3LhUKnrxa6jjWlWAtySzSV28JEBUHNFklSzES1sHsI0RcTS41eTqehgWPlqrt-nwPVpCcdH2SFXLIxxoMjWKVDszCJT-~ocylTKp1LtGNnFL3pAycgHS2kO-OPPsbKU761~qDlOKdSEjIJb26wdUhOjm5jhklFutSM8sb9lO2VQiiJyiHMgfZLq7Ny2gwmL9U-uT2jpRI1HHlHYbJ6L0WuL2A-CNzHgv6tHijBxn4dDab8iSlqx1raE7934REThQ~MO-uE41ysBMBYyTZn6gmR-EG-miLR09j4IRrHd6QztW1LJQ2JJwJustrNYooSwG-sm-dAn~omrC1mBWESyw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal