In this issue of Blood, Belle et al have identified a key role for interleukin-27 (IL-27) in acute graft-versus-host disease (GVHD), which is a complex process orchestrated by donor immune T cells and antigen-presenting cells of both donor and host origin.1
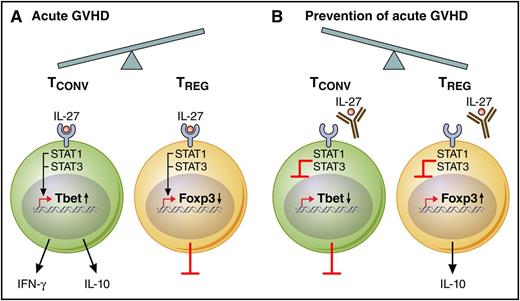
IL-27 influences the ratio of conventional T cells (TCONV) to Treg populations during GVHD. (A) During acute GVHD pathogenesis, IL-27 receptor activation induces STAT1/STAT3 signaling, which in turn promotes Tbet transcription factor expression and downstream effector function, including both interferon-γ (IFN-γ) and IL-10 secretion. The shift toward TCONV cells during acute GVHD is further exacerbated by a direct inhibitory effect of IL-27 on Treg cell in vivo stability. (B) Therapeutic mAb blockade of IL-27 reduces acute GVHD by limiting Th1- and Tc1-type TCONV cells, including a reduction in IFN-γ and IL-10 secretion; in reciprocal fashion, anti–IL-27 therapy led to a dramatic increase in inducible Treg cells with preserved IL-10 secretion. Professional illustration by Patrick Lane, ScEYEnce Studios.
IL-27 influences the ratio of conventional T cells (TCONV) to Treg populations during GVHD. (A) During acute GVHD pathogenesis, IL-27 receptor activation induces STAT1/STAT3 signaling, which in turn promotes Tbet transcription factor expression and downstream effector function, including both interferon-γ (IFN-γ) and IL-10 secretion. The shift toward TCONV cells during acute GVHD is further exacerbated by a direct inhibitory effect of IL-27 on Treg cell in vivo stability. (B) Therapeutic mAb blockade of IL-27 reduces acute GVHD by limiting Th1- and Tc1-type TCONV cells, including a reduction in IFN-γ and IL-10 secretion; in reciprocal fashion, anti–IL-27 therapy led to a dramatic increase in inducible Treg cells with preserved IL-10 secretion. Professional illustration by Patrick Lane, ScEYEnce Studios.
IL-27 is a heterodimeric, pleiotropic cytokine that somewhat paradoxically provides pro- or anti-inflammatory cues depending on the particular in vivo context.2 Given these complexities, it is noteworthy that the work of Belle et al has both dissected the role of IL-27 in acute GVHD and provided an initial rationale for IL-27 blockade as a new approach for GVHD prevention.
As summarized, Belle et al have demonstrated that genetic deficiency of the IL-27 receptor or monoclonal antibody (mAb) blockade of the IL-27p28 cytokine subunit reduces acute GVHD by shifting donor T-cell immunity away from pathogenic Tbet+ CD4+ T helper 1 (Th1) and CD8+Tc1 cells and toward CD4+ and CD8+ FoxP3-expressing regulatory T cells (Tregs) (see figure panels A-B).
The results presented by Belle et al are unique relative to some previous models that identified an anti-inflammatory role of IL-27 signaling. However, this apparent discrepancy can be resolved because such models were characterized by Th17-driven autoimmunity that was amenable to regulation by IL-27–mediated promotion of Tbet+ T cells that secreted tissue-protective IL-10.3 In an analogous manner, the severity of acute GVHD can fluctuate depending on the reciprocal balance of fate defining transcription factors4 : however, as illustrated by Belle et al., IL-27 signaling seems to only further drive the inherent predisposition of posttransplant T cells toward the pathogenic Tbet+ Th1/Tc1 subsets. In the absence of posttransplant IL-27 signaling, the drive for Tbet+ effectors was diminished, thereby allowing protective Treg cell reconstitution.
It is important to note that IL-27 represents a cytokine capable of promoting IL-10 secretion in effector T cells3 and Treg subsets.5 As such, one might have concern that therapeutic blockade of IL-27 could abrogate the known beneficial effect of Treg cell-derived IL-10 in preventing acute GVHD.6 To the contrary, Belle et al demonstrated that IL-27 therapeutic blockade not only fully preserved Treg cell IL-10 secretion but also enhanced Treg cell stability in vivo during GVHD.
In future investigations, it will be important to extend the current disease prevention studies and determine the effect of IL-27 blockade on established GVHD, which is characterized by ongoing Tbet+ T-cell–mediated inflammation in the skin, gut, and liver. In other inflammatory models, Treg cell efficacy for the treatment of inflammatory disease is dependent upon the expression of Tbet, which in turn dictates a chemokine receptor expression profile that allows Treg cell migration into the diseased tissue.7 As such, it remains to be determined whether Treg cells starved of the Tbet-inducing cytokine IL-27 will have a transcriptional machinery sufficient for infiltration into active GVHD sites.
It will also be critical to determine the effects of IL-27 blockade on the key immunologic reaction that typically necessitates allogeneic bone marrow transplantation, that is, the graft-versus-tumor (GVT) effect. On this point, there may be reason for concern, because IL-27 promotes the antitumor effect of CD8+ T cells8 ; and, IL-27 directly inhibits the growth of human acute myeloid leukemia cells.9 However, because IL-27 can increase the expression of IL-10 and programmed death ligand-1,10 it is possible that IL-27 blockade might promote antitumor effects in settings driven by these immunosuppressive factors. In conclusion, Belle et al have clearly demonstrated that IL-27 blockade can favorably skew the balance of TCONV to Treg cells for effective GVHD prevention and have set the stage for further studies to determine whether an anti–IL-27 approach can be used to treat established GVHD, or to favorably balance GVHD and GVT effects.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal