Key Points
Blockade of IL-27 signaling mitigates the severity of GVHD by recalibrating the effector and regulatory arms of the immune system.
Inhibition of IL-27 augments the reconstitution of CD4+ and CD8+ regulatory T cells and increases the stability of Foxp3 expression.
Abstract
Reestablishment of competent regulatory pathways has emerged as a strategy to reduce the severity of graft-versus-host disease (GVHD), and recalibrate the effector and regulatory arms of the immune system. However, clinically feasible, cost-effective strategies that do not require extensive ex vivo cellular manipulation have remained elusive. In the current study, we demonstrate that inhibition of the interleukin-27p28 (IL-27p28) signaling pathway through antibody blockade or genetic ablation prevented lethal GVHD in multiple murine transplant models. Moreover, protection from GVHD was attributable to augmented global reconstitution of CD4+ natural regulatory T cells (nTregs), CD4+ induced Tregs (iTregs), and CD8+ iTregs, and was more potent than temporally concordant blockade of IL-6 signaling. Inhibition of IL-27p28 also enhanced the suppressive capacity of adoptively transferred CD4+ nTregs by increasing the stability of Foxp3 expression. Notably, blockade of IL-27p28 signaling reduced T-cell–derived-IL-10 production in conventional T cells; however, there was no corresponding effect in CD4+ or CD8+ Tregs, indicating that IL-27 inhibition had differential effects on IL-10 production and preserved a mechanistic pathway by which Tregs are known to suppress GVHD. Targeting of IL-27 therefore represents a novel strategy for the in vivo expansion of Tregs and subsequent prevention of GVHD without the requirement for ex vivo cellular manipulation, and provides additional support for the critical proinflammatory role that members of the IL-6 and IL-12 cytokine families play in GVHD biology.
Introduction
Graft-versus-host disease (GVHD) is characterized by the increased production of inflammatory cytokines, activation and expansion of alloreactive donor T cells, and the failure of existing regulatory mechanisms to counterbalance this proinflammatory milieu.1-3 The latter, in particular, has been a major focus of inquiry given that GVHD is characterized by impaired reconstitution of regulatory T cells (Tregs) which contributes substantially to the pathophysiology of this disease.4-6 This observation has been the impetus for strategies directed at the reestablishment of an effective Treg network by the adoptive transfer of ex vivo–expanded Tregs.7-9 Although these studies have demonstrated feasibility, there have been no controlled studies demonstrating efficacy, and the technology necessary for this approach is not widely available to all transplant centers.10 Thus, alternative strategies designed to facilitate the in vivo expansion of existing Treg populations by modulating the inflammatory cytokine milieu via antibody blockade11,12 or exogenous cytokine administration13 have intrinsic merit given the potential broader clinical availability of these approaches.
Interleukin-6 (IL-6), along with other IL-6 cytokine superfamily members such as IL-23, has been shown to have an important proinflammatory role in GVHD in both preclinical murine models11,14-16 and recent clinical studies.17,18 IL-27, another member of the IL-6 cytokine family, is a heterodimeric cytokine that is composed of p28 and Epstein-Barr–induced gene 3 (EBI3) subunits and signals through a heterodimeric receptor composed of WSX-1 and gp13019 which is part of the IL-6 signaling complex.20 Like IL-23, IL-27 is secreted by activated antigen-presenting cells (APCs) such as macrophages, monocytes, and dendritic cells and signals through Stat3.21 The IL-27R is highly expressed on effector memory CD4+ and CD8+ T cells,22 and ligation of the receptor leads to Stat1 and Stat3 activation.23 Although initially thought to have proinflammatory effects, more recent studies have uncovered an immunoregulatory role for IL-27 which has been derived from data showing that IL-27 suppresses retinoid-related orphan receptor γt (RORγt) T helper 17 (TH17) development24 and increases T-cell production of IL-10.25 Notably, IL-27 has also been shown to affect Treg biology, although whether IL-27 inhibits or enhances Treg expansion remains controversial and appears to be dependent, in part, upon the experimental conditions.19,22,26-29 The goal of the current report therefore was to determine whether IL-27 exerted proinflammatory or immune-suppressive effects during GVHD, and to examine specifically the effect of IL-27 on the reconstitution of the Treg compartment under these inflammatory conditions.
Methods
Mice
C57BL/6 (B6) (H-2b), Balb/c (H-2d), Balb.B (H-2b), and B6 Foxp3EGFP mice were bred in the Animal Resource Center (ARC) at the Medical College of Wisconsin (MCW) or purchased from The Jackson Laboratory (Bar Harbor, ME). IL-27p28−/−, IL-27R−/−, and Foxp3ΔEGFP mice in which there is mutation in the Foxp3 coding region which renders the Foxp3 gene nonfunctional have been described.24,30,31 IL-10BiT-Foxp3EGFP reporter mice were kindly provided by Dr Casey Weaver (University of Alabama Birmingham, Birmingham, AL).32 IL-27R−/−Foxp3EGFP animals were made by intercrossing IL-27R−/− × Foxp3EGFP heterozygotes and screening for homozygosity by polymerase chain reaction.
Reagents
Anti-IL-27 (p28) (MM27.7B1) is a previously described mouse immunoglobulin G2 (IgG2) antibody.33 Animals received 0.5 mg intraperitoneally on days 0 and 6 posttransplantation. Mouse IgG2a (BioXCell, West Lebanon, NH) was used as a control and administered at the same dose and schedule. Anti-IL-6R antibody (MR-16-1) is a rat IgG antibody.11 Animals received a loading dose of 2 mg IV on day 0, and then 0.5 mg on day 7 by intraperitoneal injection. Rat IgG (Jackson ImmunoResearch Laboratories, West Grove, PA) was used as a control for MR-16-1.
Other detailed methods
All other methods are described in supplemental Methods (available on the Blood Web site).
Results
Transplantation with marrow grafts from IL-27p28−/− mice exacerbates GVHD lethality
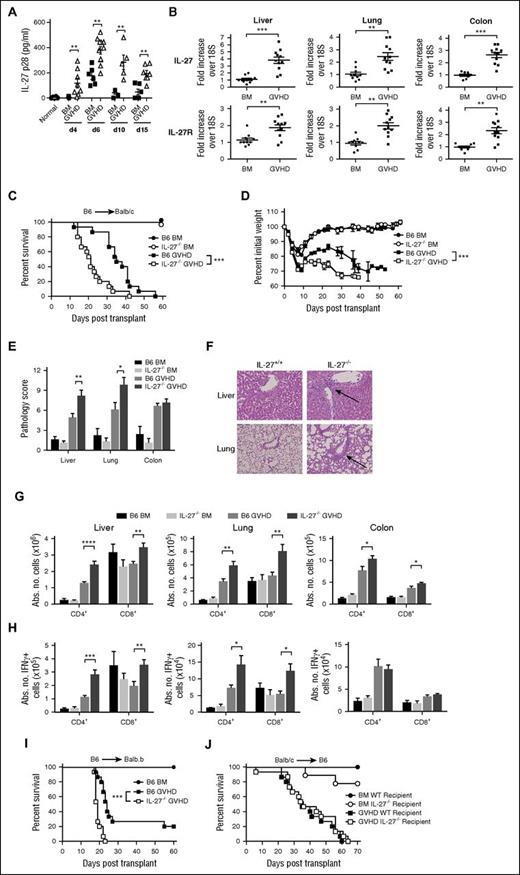
IL-27 is a member of the IL-6 cytokine superfamily which includes IL-12 and IL-23. However, in contrast to these cytokines, which have predominantly inflammatory roles, IL-27 has both inflammatory and immunoregulatory effects in nontransplant models.19,21,34 To define the role of IL-27 in GVHD, we first observed that plasma IL-27 levels were significantly increased in GVHD animals peaking at day 6 (Figure 1A), and that IL-27 and IL-27R messenger RNA levels were augmented in the liver, lung, and colon compared with bone marrow (BM) control animals (Figure 1B). Because IL-27 is secreted by APCs35 and has effects on T cells,22,36 both donor and recipient-derived IL-27 have the potential to modulate GVHD severity. We observed that transplantation with IL-27p28−/− donor grafts exacerbated GVHD mortality (Figure 1C) and accelerated weight loss (Figure 1D) compared with animals that received wild-type grafts. Histological analysis revealed that there was increased pathological damage in the liver and lungs of these animals (Figure 1E). A characteristic in both liver and lung was the finding of increased numbers of inflammatory infiltrates in periportal and perivascular areas, respectively (Figure 1F). There was also an increased number of donor-derived CD4+ and CD8+ T cells in liver, lung, and colon, as well as a significant increase in CD4+ and CD8+ interferon-γ–positive (IFN-γ+) T cells in the liver and lung of recipients of IL-27p28−/− marrow grafts (Figure 1G-H). Conversely, there was no difference in the absolute number of Tregs in the spleen, liver, colon, or lung (supplemental Figure 1), indicative of an imbalance between the effector and regulatory arms of the immune system. We also observed that animals transplanted with wild-type as opposed to IL-27p28−/− BM had worse survival consistent with the premise that APC-derived IL-27 was most critical for exacerbating GVHD (supplemental Figure 2). A similar survival outcome was observed in a major histocompatibility complex–matched, minor antigen mismatched GVHD model (B6→Balb.B) (Figure 1I). Notably, there was no difference in survival between B6 and IL-27p28−/− recipient animals reconstituted with wild-type Balb/c grafts, indicating that host-derived IL-27 had no substantial impact on GVHD mortality (Figure 1J).
Transplantation with IL-27p28−/− marrow grafts exacerbates GVHD. (A) Lethally irradiated (900 cGy) Balb/c mice were transplanted with B6 BM alone (▪, n = 5-6 per time point) or together with B6 spleen cells (adjusted to yield an αβ T-cell dose of 0.7 × 106) (△, n = 5-11 per time point). Normal nontransplanted Balb/c mice served as controls (●, n = 6). Plasma IL-27p28 levels are depicted at each time point in individual animals. (B) IL-27 and IL-27R messenger RNA expression in the liver, lung, and colon of Balb/c mice transplanted with B6 BM alone (5 × 106) (●, n = 10) or B6 BM and B6 spleen cells (adjusted to yield an αβ T-cell dose of 0.3 × 106) (▪, n = 11) 3 weeks posttransplantation. (C-D) Lethally irradiated (900 cGy) Balb/c recipients were transplanted with B6 BM alone (●, n = 9), IL-27−/− BM alone (○, n = 9), B6 BM and B6 spleen cells (▪, n = 15), or IL-27−/− BM and spleen cells (□, n = 15) (adjusted to yield an αβ T-cell dose of 0.6 × 106 cells). Overall survival and serial weight curves are depicted. (E) Pathological scores of liver, lung, and colon from Balb/c mice transplanted with B6 BM (black bar), IL-27−/− BM (light gray bar), B6 BM and spleen cells (medium gray bar), or IL-27−/− BM and spleen cells (dark gray bar) 14 days posttransplantation. Data are from 6 to 12 mice per group. (F) Representative hematoxylin and eosin–stained sections of the lung and liver of animals transplanted with B6 BM and spleen cells or IL-27−/− BM and spleen cells as in panel E. Arrows denote areas of periportal and perivascular lymphocytic infiltration. Original magnification is ×50 for photomicrographs. (G-H) Lethally irradiated Balb/c recipients were transplanted with B6 BM alone (black bars, n = 9), IL-27−/− BM (light gray bars, n = 9), B6 BM and B6 spleen cells (medium gray bars, n = 15), or IL-27−/− BM and spleen cells (dark gray bars, n = 15). The absolute number (Abs No) of donor-derived CD4+ and CD8+ T cells (G) and CD4+ and CD8+ T cells that secreted IFN-γ (H) in the liver, lung, and colon 14 days posttransplantation is depicted. (I) Lethally irradiated (900 cGy) Balb.B mice were transplanted with B6 BM alone (●, n = 9), B6 BM and B6 spleen cells (▪, n = 15), or IL-27−/− BM and spleen cells (□, n = 15) (adjusted to yield an αβ T-cell dose of 6 × 106 cells). (J) Lethally irradiated (1100 cGy) B6 (▪, n = 15) or IL-27−/− (□, n = 15) animals were transplanted with Balb/c BM and spleen cells (adjusted to a dose of 4.8 × 106 αβ T cells). B6 (●, n = 9) or IL-27−/− (○, n = 9) mice reconstituted with Balb/c BM alone served as controls. Overall survival is depicted. Results are from 2 to 3 experiments in all panels. *P < .05, **P < .01, ***P < .001.
Transplantation with IL-27p28−/− marrow grafts exacerbates GVHD. (A) Lethally irradiated (900 cGy) Balb/c mice were transplanted with B6 BM alone (▪, n = 5-6 per time point) or together with B6 spleen cells (adjusted to yield an αβ T-cell dose of 0.7 × 106) (△, n = 5-11 per time point). Normal nontransplanted Balb/c mice served as controls (●, n = 6). Plasma IL-27p28 levels are depicted at each time point in individual animals. (B) IL-27 and IL-27R messenger RNA expression in the liver, lung, and colon of Balb/c mice transplanted with B6 BM alone (5 × 106) (●, n = 10) or B6 BM and B6 spleen cells (adjusted to yield an αβ T-cell dose of 0.3 × 106) (▪, n = 11) 3 weeks posttransplantation. (C-D) Lethally irradiated (900 cGy) Balb/c recipients were transplanted with B6 BM alone (●, n = 9), IL-27−/− BM alone (○, n = 9), B6 BM and B6 spleen cells (▪, n = 15), or IL-27−/− BM and spleen cells (□, n = 15) (adjusted to yield an αβ T-cell dose of 0.6 × 106 cells). Overall survival and serial weight curves are depicted. (E) Pathological scores of liver, lung, and colon from Balb/c mice transplanted with B6 BM (black bar), IL-27−/− BM (light gray bar), B6 BM and spleen cells (medium gray bar), or IL-27−/− BM and spleen cells (dark gray bar) 14 days posttransplantation. Data are from 6 to 12 mice per group. (F) Representative hematoxylin and eosin–stained sections of the lung and liver of animals transplanted with B6 BM and spleen cells or IL-27−/− BM and spleen cells as in panel E. Arrows denote areas of periportal and perivascular lymphocytic infiltration. Original magnification is ×50 for photomicrographs. (G-H) Lethally irradiated Balb/c recipients were transplanted with B6 BM alone (black bars, n = 9), IL-27−/− BM (light gray bars, n = 9), B6 BM and B6 spleen cells (medium gray bars, n = 15), or IL-27−/− BM and spleen cells (dark gray bars, n = 15). The absolute number (Abs No) of donor-derived CD4+ and CD8+ T cells (G) and CD4+ and CD8+ T cells that secreted IFN-γ (H) in the liver, lung, and colon 14 days posttransplantation is depicted. (I) Lethally irradiated (900 cGy) Balb.B mice were transplanted with B6 BM alone (●, n = 9), B6 BM and B6 spleen cells (▪, n = 15), or IL-27−/− BM and spleen cells (□, n = 15) (adjusted to yield an αβ T-cell dose of 6 × 106 cells). (J) Lethally irradiated (1100 cGy) B6 (▪, n = 15) or IL-27−/− (□, n = 15) animals were transplanted with Balb/c BM and spleen cells (adjusted to a dose of 4.8 × 106 αβ T cells). B6 (●, n = 9) or IL-27−/− (○, n = 9) mice reconstituted with Balb/c BM alone served as controls. Overall survival is depicted. Results are from 2 to 3 experiments in all panels. *P < .05, **P < .01, ***P < .001.
Antibody blockade of IL-27 reduces GVHD severity and prolongs survival
To confirm the results observed with donor IL-27p28−/− mice in a more clinically relevant manner, we conducted similar studies using an IL-27p28–specific antibody. To determine whether p28 antibody was linked to EBI3 and therefore capable of detecting IL-27, we examined the ability of an anti-EBI3 antibody (MEBI3.B1) to remove the p28 signal that we detected in the plasma of GVHD mice (Figure 1A). MEBI3-B1was shown to be specific for EBI3 as it bound to mouse EBI3, mouse IL-27, and human IL-27, but not mouse p28 (supplemental Figure 3A). Incubation of GVHD plasma on immobilized anti-EBI3–coated plates revealed a significant reduction in the p28 signal (supplemental Figure 3B), indicating that most of the p28 that was detected was bound to EBI3 and therefore IL-27, although we cannot exclude the possibility that a small percentage of p28 could also complex with another as-yet-unidentified subunit. In subsequent studies, mice were administered p28 antibody on days 0 and 6 to coincide with peak IL-27 levels detected in the plasma during GVHD. In contrast to what was observed with IL-27p28−/− donors, recipients treated with an anti-IL-27p28–specific antibody had significantly improved survival (Figure 2A) and weight recovery (Figure 2B) when compared with isotype antibody-treated mice. Pathological damage in the liver, lung, and colon was also significantly reduced (Figure 2C-D). There was a corresponding reduction in the number of donor-derived CD4+ and CD8+ T cells in the liver, lung, and colon of p28 antibody-treated mice (Figure 2E), as well as a significant decrease in CD4+ and CD8+ IFN-γ+ T cells (Figure 2F). Administration of p28 antibody also significantly improved survival (Figure 2G) and weight recovery (Figure 2H) in B6→Balb.B recipients. Finally, we observed that p28 antibody treatment significantly attenuated GVHD mortality in recipients of IL-27p28−/− marrow grafts, indicative of efficacy even under more severe GVHD conditions (supplemental Figure 4). Collectively, these results indicated that antibody blockade of IL-27p28 signaling attenuated GVHD severity and reduced mortality, in contrast to results observed with transplantation with IL-27p28−/− grafts.
Antibody blockade of IL-27p28 signaling mitigates the severity of GVHD. (A-B) Irradiated Balb/c recipients were transplanted with B6 BM alone (●, n = 9), or B6 BM and B6 spleen cells (adjusted to yield an αβ T-cell dose of 0.6 × 106 cells) and treated with either an isotype (Iso) control (▪, n = 15) or p28 antibody (□, n = 15). Overall survival and serial weight curves are depicted. (C) Pathological scores of liver, lung, and colon from Balb/c mice transplanted with B6 BM (black bars, n = 8), or B6 BM and B6 spleen cells and treated with either isotype control (light gray bars, n = 15) or p28 antibody (dark gray bars, n = 13-15) 4 weeks posttransplantation. (D) Representative hematoxylin and eosin–stained sections of the liver, lung, and colon from animals transplanted with B6 BM and spleen cells and then treated with isotype control or p28 antibody as in panel C. Arrows denote areas of periportal and perivascular lymphocytic infiltration, respectively. Original magnification is ×50 for liver and lung, and ×100 for colon photomicrographs. (E-F) Balb/c recipients were transplanted with B6 BM alone (black bars, n = 6-8) or B6 BM and B6 spleen cells and treated with either an isotype control (light gray bars, n = 14-15) or p28 antibody (dark gray bars, n = 14-15). The absolute number of donor-derived CD4+ and CD8+ T cells (E) and CD4+ and CD8+ T cells that secreted IFN-γ (F) in the liver, lung, and colon 14 days posttransplantation is depicted. (G-H) Lethally irradiated Balb.B mice were transplanted with B6 BM alone (●, n = 9) or B6 BM and B6 spleen cells (adjusted to yield an αβ T-cell dose of 6 × 106 cells) and then treated with an isotype control (▪, n = 15) or p28 (□, n = 15) antibody. Overall survival and serial weight curves are depicted. Results are from 2 to 3 experiments in all panels. *P < .05, **P < .01, ***P < .001.
Antibody blockade of IL-27p28 signaling mitigates the severity of GVHD. (A-B) Irradiated Balb/c recipients were transplanted with B6 BM alone (●, n = 9), or B6 BM and B6 spleen cells (adjusted to yield an αβ T-cell dose of 0.6 × 106 cells) and treated with either an isotype (Iso) control (▪, n = 15) or p28 antibody (□, n = 15). Overall survival and serial weight curves are depicted. (C) Pathological scores of liver, lung, and colon from Balb/c mice transplanted with B6 BM (black bars, n = 8), or B6 BM and B6 spleen cells and treated with either isotype control (light gray bars, n = 15) or p28 antibody (dark gray bars, n = 13-15) 4 weeks posttransplantation. (D) Representative hematoxylin and eosin–stained sections of the liver, lung, and colon from animals transplanted with B6 BM and spleen cells and then treated with isotype control or p28 antibody as in panel C. Arrows denote areas of periportal and perivascular lymphocytic infiltration, respectively. Original magnification is ×50 for liver and lung, and ×100 for colon photomicrographs. (E-F) Balb/c recipients were transplanted with B6 BM alone (black bars, n = 6-8) or B6 BM and B6 spleen cells and treated with either an isotype control (light gray bars, n = 14-15) or p28 antibody (dark gray bars, n = 14-15). The absolute number of donor-derived CD4+ and CD8+ T cells (E) and CD4+ and CD8+ T cells that secreted IFN-γ (F) in the liver, lung, and colon 14 days posttransplantation is depicted. (G-H) Lethally irradiated Balb.B mice were transplanted with B6 BM alone (●, n = 9) or B6 BM and B6 spleen cells (adjusted to yield an αβ T-cell dose of 6 × 106 cells) and then treated with an isotype control (▪, n = 15) or p28 (□, n = 15) antibody. Overall survival and serial weight curves are depicted. Results are from 2 to 3 experiments in all panels. *P < .05, **P < .01, ***P < .001.
CD8+ T cells from IL-27−/− mice are inherently biased toward a proinflammatory phenotype which exacerbates GVHD
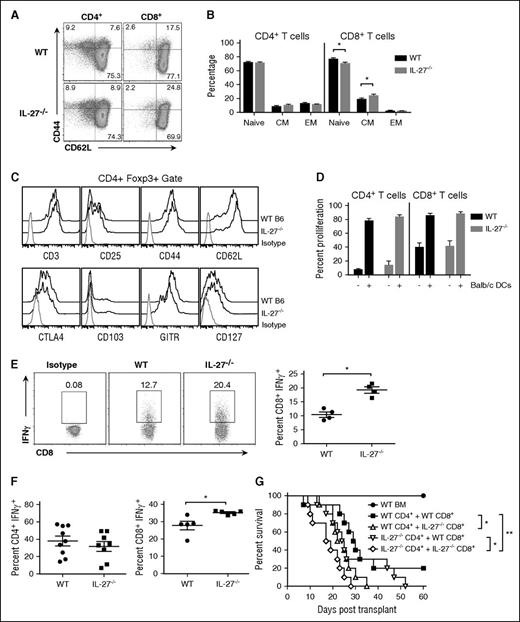
This discordance in results was unexpected and led us to consider whether there were baseline alterations in transgenic T cells from IL-27−/− animals that would bias these cells toward a more inflammatory phenotype. To determine whether this premise was correct, we performed phenotypic characterization and functional assessment on T cells from both wild-type and IL-27p28−/− mice. We observed a significant increase in the percentage of central memory CD8+, but not CD4+, T cells in the spleens of IL-27−/− mice and a corresponding reduction in the frequency of naive CD8+ T cells (Figure 3A-B). Phenotypic examination of CD4+Foxp3+ T cells revealed no difference in expression of CD25, CD44, CD62L, CTLA4, CD103, glucocorticoid-induced tumor necrosis factor (TNF) receptor–related protein (GITR), and CD127 (Figure 3C). In functional studies, there was no difference in proliferation when T cells from IL-27−/− mice were cultured in the presence of allogeneic APCs (Figure 3D). However, when splenic T cells were stimulated in vitro with phorbol 12-myristate 13-acetate (PMA)/ionomycin, we observed an increased percentage of CD8+, but not CD4+, T cells (data not shown), which produced IFN-γ from IL-27p28−/− mice (Figure 3E). There was no difference in the percentage of either CD4+ or CD8+ T cells that expressed other inflammatory cytokines such as TNF-α and granulocyte-macrophage colony-stimulating factor (GM-CSF), and an actual decrease in IL-2, after similar polyclonal activation (supplemental Figure 5A). Culture of CD4+ and CD8+ T cells under TH1 polarizing conditions also revealed a significant increase in the percentage of CD8+IFN-γ+, but not CD4+IFN-γ+, T cells from IL-27p28−/− animals (Figure 3F). TH1-polarized CD8+ T cells from IL-27p28−/− animals also had increased expression of T-bet (supplemental Figure 5B). To determine whether CD8+ T cells from IL-27p28−/− animals could exacerbate GVHD, we performed T-cell subset transfer experiments in which animals were reconstituted with CD8+ and/or CD4+ T cells from either wild-type or IL-27p28−/− animals. Mice that were transplanted with wild-type CD4+ and IL-27p28−/−CD8+ T cells had significantly worse survival compared with animals reconstituted with wild-type CD4+ and CD8+ T cells (Figure 3G). In contrast, transplantation with IL-27p28−/−CD4+ T cells and wild-type CD8+ T cells did not increase GVHD lethality relative to mice reconstituted with wild-type CD4+ and CD8+ T cells. Collectively, these results were evidence that the lack of endogenous IL-27 resulted in intrinsic immune dysregulation in CD8+ T cells which augmented their ability to exacerbate GVHD.
CD8+ T cells from IL-27−/− mice have a biased inflammatory phenotype. (A) Representative dot plots depicting CD44 and CD62L expression on CD4+ and CD8+ T cells from B6 and IL-27−/− mice. (B) Percentage of CD4+ and CD8+ T cells from wild-type (WT) or IL-27−/− animals (n = 9 per group) with a naive, central memory (CM), or effector memory (EM) phenotype. (C) Representative histograms showing CD25, CD44, CD62L, CTLA4, CD103, GITR, and CD127 expression on gated CD4+Foxp3+ T cells from wild-type or IL-27−/− mice. (D) Column-purified T cells (1 × 105) from wild-type or IL-27−/− mice were labeled with carboxyfluorescein diacetate succinimidyl ester (CFSE) and cultured with allogeneic Balb/c CD11c-enriched dendritic cells (DCs) (5 × 104) for 4 days. The percentage of CFSEloCD4+ and CD8+ T cells in the absence or presence of DCs is shown for replicate experiments (n = 3). Data are presented as mean ± standard error of the mean (SEM). (E) Splenocytes from wild-type or IL-27−/− mice were cultured with PMA and ionomycin for 6 hours. Representative dot plots depicting the percentage of CD8+ T cells that secreted IFN-γ and scatterplots for replicate experiments (n = 4) are shown. (F) Purified CD4+ or CD8+ T cells (1 × 105) from the spleens of wild-type or IL-27−/− mice were cultured under TH1 polarizing conditions (see “Methods”) for 5 days. Scatterplots depicting the percentage of CD4+ or CD8+ IFN-γ+ T cells are shown (n = 5-9 per group). (G) Lethally irradiated Balb/c mice were transplanted with B6 BM alone (●, n = 6), or together with either B6 CD4+ (0.9 × 106) and CD8+ (0.5 × 106) T cells (▪, n = 10), B6 CD4+ and IL-27−/− CD8+ T cells (△, n = 10), IL-27−/− CD4+ and B6 CD8+ T cells (▿, n = 10), or IL-27−/− CD4+ and CD8+ T cells (♢, n = 10). Survival is shown. Data are from 2 experiments. *P < .05, **P < .01.
CD8+ T cells from IL-27−/− mice have a biased inflammatory phenotype. (A) Representative dot plots depicting CD44 and CD62L expression on CD4+ and CD8+ T cells from B6 and IL-27−/− mice. (B) Percentage of CD4+ and CD8+ T cells from wild-type (WT) or IL-27−/− animals (n = 9 per group) with a naive, central memory (CM), or effector memory (EM) phenotype. (C) Representative histograms showing CD25, CD44, CD62L, CTLA4, CD103, GITR, and CD127 expression on gated CD4+Foxp3+ T cells from wild-type or IL-27−/− mice. (D) Column-purified T cells (1 × 105) from wild-type or IL-27−/− mice were labeled with carboxyfluorescein diacetate succinimidyl ester (CFSE) and cultured with allogeneic Balb/c CD11c-enriched dendritic cells (DCs) (5 × 104) for 4 days. The percentage of CFSEloCD4+ and CD8+ T cells in the absence or presence of DCs is shown for replicate experiments (n = 3). Data are presented as mean ± standard error of the mean (SEM). (E) Splenocytes from wild-type or IL-27−/− mice were cultured with PMA and ionomycin for 6 hours. Representative dot plots depicting the percentage of CD8+ T cells that secreted IFN-γ and scatterplots for replicate experiments (n = 4) are shown. (F) Purified CD4+ or CD8+ T cells (1 × 105) from the spleens of wild-type or IL-27−/− mice were cultured under TH1 polarizing conditions (see “Methods”) for 5 days. Scatterplots depicting the percentage of CD4+ or CD8+ IFN-γ+ T cells are shown (n = 5-9 per group). (G) Lethally irradiated Balb/c mice were transplanted with B6 BM alone (●, n = 6), or together with either B6 CD4+ (0.9 × 106) and CD8+ (0.5 × 106) T cells (▪, n = 10), B6 CD4+ and IL-27−/− CD8+ T cells (△, n = 10), IL-27−/− CD4+ and B6 CD8+ T cells (▿, n = 10), or IL-27−/− CD4+ and CD8+ T cells (♢, n = 10). Survival is shown. Data are from 2 experiments. *P < .05, **P < .01.
Transplantation with IL-27R−/− marrow grafts reduces GVHD severity and accumulation of proinflammatory T cells in target organs
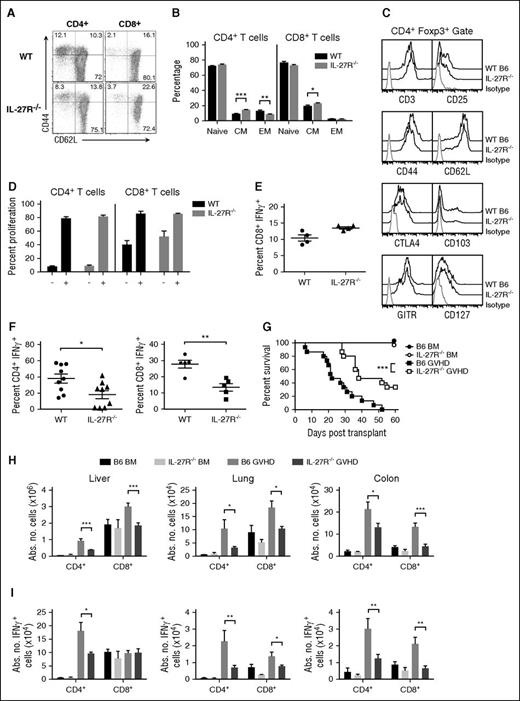
To provide additional confirmation that the protective effects observed with p28 antibody blockade were valid, we used IL-27R−/− (WSX-1−/−) animals in which there is specific deletion of the unique component of the heterodimeric IL-27R.29 Phenotypic characterization revealed that both CD4+ and CD8+ T cells from IL-27R−/− mice were also biased toward a central memory phenotype (Figure 4A-B) and that CD4+Foxp3+ Tregs were phenotypically similar to wild-type Tregs (Figure 4C). Functional studies revealed no difference when compared with wild-type T cells with respect to alloantigen-induced proliferation or CD8+ IFN-γ production (Figure 4D-E). There was, however, a modest reduction in the percentage of CD4+GM-CSF+ and CD8+TNF-α+ T cells after polyclonal stimulation (supplemental Figure 6A). More significantly, both CD4+ and CD8+ T cells from IL-27R−/− mice also produced less IFN-γ, and CD8+ T cells had reduced T-bet expression after TH1 polarization (Figure 4F; supplemental Figure 6B), consistent with the described role of IL-27 in promoting TH1 responses.37 We observed that transplantation with marrow grafts from IL-27R−/− mice resulted in significantly prolonged survival compared with wild-type control animals (Figure 4G). There was also a significant reduction in the absolute number of total CD4+ and CD8+ T cells, as well as CD4+ and CD8+ IFN-γ+ T cells, in the liver, lung, and colon (Figure 4H-I). Animals transplanted with IL-27R−/− BM and wild-type spleen cells had significantly worse survival compared with mice reconstituted with IL-27R−/− BM and IL-27R−/− spleen cells (supplemental Figure 7A). Conversely, IL-27R expression on BM alone had no adverse effect on overall survival (supplemental Figure 7B), indicating that IL-27R expression on T cells, and not BM cells, was critical for driving GVHD. Collectively, these studies demonstrated that transplantation with IL-27R−/−, in contrast to IL-27p28−/−, marrow grafts resulted in protection from lethal GVHD which supported the findings observed after p28 antibody blockade.
Transplantation with IL-27R−/− marrow grafts reduces GVHD severity. (A) Representative dot plots depicting CD44 and CD62L expression on CD4+ and CD8+ T cells from B6 and IL-27R−/− mice. (B) Percentage of CD4+ and CD8+ T cells from wild-type or IL-27R−/− animals (n = 10 per group) with a naive, central memory (CM), or effector memory (EM) phenotype. (C). Representative dot plot showing CD25, CD44, CD62L, CTLA4, CD103, GITR, and CD127 expression on gated CD4+ Foxp3+ T cells from wild-type or IL-27R−/− mice. (D). Column-purified T cells (1 × 105) from wild-type or IL-27R−/− mice were labeled with CFSE and cultured with allogeneic Balb/c CD11c-enriched dendritic cells (DCs) (5 × 104) for 4 days. The percentage of CFSElo CD4+ and CD8+ T cells in the absence or presence of DCs is shown for replicate experiments (n = 3). Data are presented as mean ± SEM. (E). Column-purified T cells (3 × 106) from wild-type or IL-27R−/− mice were cultured with PMA and ionomycin for 6 hours. Representative dot plots depicting the percentage of CD8+ T cells that secreted IFN-γ and scatterplots for replicate experiments (n = 4) are shown. (F). Purified CD4+ or CD8+ T cells from the spleens of wild-type or IL-27R−/− mice were cultured under TH1 polarizing conditions for 5 days. Scatterplots depicting the percentage of CD4+ or CD8+ IFN-γ+ T cells are shown (n = 5-9 per group). (G) Balb/c recipients were transplanted with B6 BM alone (●, n = 9), IL-27R−/− BM (○, n = 9), B6 BM and B6 spleen cells (▪, n = 15), or IL-27R−/− BM and IL-27R−/− spleen cells (□, n = 15) (adjusted to yield an αβ T-cell dose of 0.6 × 106 cells). Overall survival is depicted. Data are from 3 experiments. (H-I) Lethally irradiated Balb/c recipients were transplanted with B6 BM alone (black bars, n = 5), IL-27R−/− BM (light gray bars, n = 5), B6 BM and B6 spleen cells (medium gray bars, n = 10), or IL-27R−/− BM and spleen cells (dark gray bars, n = 10). The absolute number of donor-derived CD4+ and CD8+ T cells (H) and CD4+ and CD8+ T cells that secreted IFN-γ (I) in the liver, lung, and colon 14 days posttransplantation is depicted. Data are from 2 experiments. *P < .05, **P < .01, ***P < .001.
Transplantation with IL-27R−/− marrow grafts reduces GVHD severity. (A) Representative dot plots depicting CD44 and CD62L expression on CD4+ and CD8+ T cells from B6 and IL-27R−/− mice. (B) Percentage of CD4+ and CD8+ T cells from wild-type or IL-27R−/− animals (n = 10 per group) with a naive, central memory (CM), or effector memory (EM) phenotype. (C). Representative dot plot showing CD25, CD44, CD62L, CTLA4, CD103, GITR, and CD127 expression on gated CD4+ Foxp3+ T cells from wild-type or IL-27R−/− mice. (D). Column-purified T cells (1 × 105) from wild-type or IL-27R−/− mice were labeled with CFSE and cultured with allogeneic Balb/c CD11c-enriched dendritic cells (DCs) (5 × 104) for 4 days. The percentage of CFSElo CD4+ and CD8+ T cells in the absence or presence of DCs is shown for replicate experiments (n = 3). Data are presented as mean ± SEM. (E). Column-purified T cells (3 × 106) from wild-type or IL-27R−/− mice were cultured with PMA and ionomycin for 6 hours. Representative dot plots depicting the percentage of CD8+ T cells that secreted IFN-γ and scatterplots for replicate experiments (n = 4) are shown. (F). Purified CD4+ or CD8+ T cells from the spleens of wild-type or IL-27R−/− mice were cultured under TH1 polarizing conditions for 5 days. Scatterplots depicting the percentage of CD4+ or CD8+ IFN-γ+ T cells are shown (n = 5-9 per group). (G) Balb/c recipients were transplanted with B6 BM alone (●, n = 9), IL-27R−/− BM (○, n = 9), B6 BM and B6 spleen cells (▪, n = 15), or IL-27R−/− BM and IL-27R−/− spleen cells (□, n = 15) (adjusted to yield an αβ T-cell dose of 0.6 × 106 cells). Overall survival is depicted. Data are from 3 experiments. (H-I) Lethally irradiated Balb/c recipients were transplanted with B6 BM alone (black bars, n = 5), IL-27R−/− BM (light gray bars, n = 5), B6 BM and B6 spleen cells (medium gray bars, n = 10), or IL-27R−/− BM and spleen cells (dark gray bars, n = 10). The absolute number of donor-derived CD4+ and CD8+ T cells (H) and CD4+ and CD8+ T cells that secreted IFN-γ (I) in the liver, lung, and colon 14 days posttransplantation is depicted. Data are from 2 experiments. *P < .05, **P < .01, ***P < .001.
Blockade of IL-27p28 signaling augments the reconstitution of Tregs
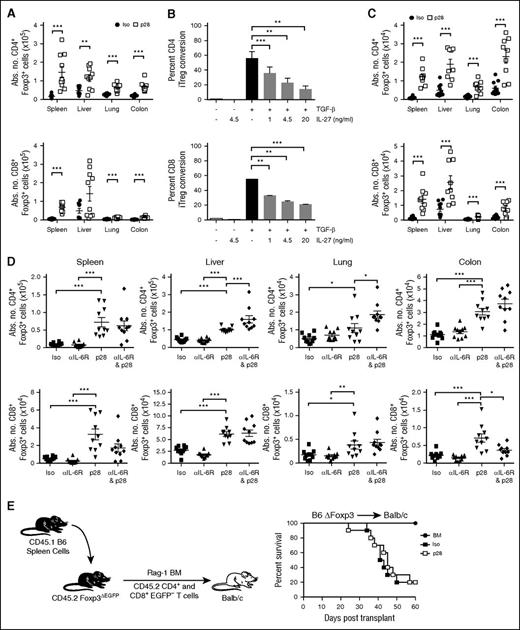
We had demonstrated that antibody blockade of the IL-6R, which shares the gp130 subunit with IL-27, prevented GVHD by augmenting Treg reconstitution.11 We therefore conducted studies to determine whether protection by IL-27p28 antibody blockade similarly affected Treg recovery. Using Foxp3EGFP reporter animals, we observed a significant increase in the absolute number of CD4+ and CD8+ Foxp3+ Tregs in all tissue sites of mice treated with p28 antibody with the exception of CD8+ Tregs in the liver (Figure 5A). Although the CD4+ Treg compartment is composed of both natural Tregs (nTregs) and induced Tregs (iTregs), nTregs constitute the majority of this compartment. We therefore examined the effect of IL-27 on the induction of Tregs from the conventional T-cell pool. In vitro assays demonstrated that IL-27 effected a dose-dependent reduction in the conversion of CD4+ and CD8+ Foxp3+ Tregs from polyclonally stimulated CD4+ and CD8+ Foxp3EGFP− T cells, respectively, indicating that IL-27 suppressed iTreg generation (Figure 5B). To determine whether this was operative in vivo, allogeneic recipients were transplanted with Rag-1 BM alone or together with sorted CD4+Foxp3EGFP− T cells and then treated with an isotype control or p28 antibody. Mice administered p28 antibody had a significantly increased number of both CD4+ and CD8+ Foxp3+ iTregs in all tissue sites, indicating that inhibition of IL-27 enhanced iTreg reconstitution (Figure 5C). We then determined whether coblockade of IL-6 and IL-27 by antibody administration had any additive effects. These studies confirmed that administration of p28 antibody enhanced Treg reconstitution, whereas anti-IL-6R antibody treatment resulted in no increase in the absolute number of CD4+ or CD8+ Tregs. Thus, blockade of IL-27 was more potent than inhibition of IL-6 for enhancing Treg reconstitution when temporally concordant schedules were used. Notably, coadministration of both antibodies had no additive effect on CD8+ Treg numbers, but did result in a significant increase in CD4+ Tregs in the liver and lung compared with p28 antibody alone (Figure 5D). Finally, we conducted studies to determine whether the salutary effects of anti-IL-27p28 antibody blockade were attributable solely to the increase in Treg reconstitution because it was formally possible that IL-27 had effects on non-Treg populations. To address this question, recipients were transplanted with sorted CD4+ and CD8+ T cells from Foxp3ΔEGFP mice which have no functional Tregs.29 We observed no difference in survival, indicating that the protective effect conferred by p28 antibody blockade was abrogated in the absence of functional Tregs (Figure 5E). Thus, these data were evidence that GVHD protection from p28 antibody administration was attributable to augmented Treg reconstitution.
Blockade of IL-27 signaling augments reconstitution of Foxp3-expressing Tregs. (A) Balb/c mice were transplanted with B6 Foxp3EGFP BM and spleen cells and then treated with either an isotype control (●) or p28 (□) antibody. Animals were killed 14 days after transplantation and the absolute number of CD4+ and CD8+ Foxp3+ T cells in the spleen, liver, lung, and colon are shown. (B) Sorted CD4+ or CD8+ Foxp3EGFP− T cells were cultured with anti-CD3/28 antibodies, IL-2, and TGF-β alone, or together with graded doses of IL-27. The percentage of CD4+ and CD8+ Foxp3+ T cells is depicted. (C) Balb/c mice were transplanted with B6 Rag-1 BM alone or together with sorted CD4+ (0.4 × 106) and CD8+ (0.2 × 106) Foxp3EGFP− T cells and then treated with either an isotype control (●) or p28 (□) antibody. Animals were killed 14 days posttransplantation and the absolute number of CD4+ and CD8+ Foxp3+ T cells in spleen, liver, lung, and colon is shown. (D) Balb/c mice were transplanted with B6 Foxp3GFP BM and spleen cells and then treated with either an isotype control (▪, n = 10), anti-IL-6R (▲, n = 10), p28 (▼, n = 10), or combination of p28 and anti-IL-6R (♦, n = 10) antibodies. The absolute number of CD4+ and CD8+ Foxp3+ T cells in spleen, liver, lung, and colon 2 weeks posttransplantation is depicted. (E) Balb/c mice transplanted with B6 Rag-1 BM alone (●, n = 5) or with 0.5 × 106 CD4+EGFP− and 0.3 × 106 CD8+EGFP− T cells from Foxp3ΔEGFP animals that had been reconstituted with 40 × 106 to 60 × 106 spleen cells from B6.SJL Foxp3EGFP (CD45.1) animals 1 to 2 days after birth to prevent the development of lethal autoimmunity. Mice transplanted with these T cells were treated with either isotype (▪, n = 10) or p28 antibody (□, n = 10) on days 0 and 6 posttransplantation. Overall survival is depicted. Data are from 2 experiments for each panel. *P < .05, **P < .01, ***P < .001. EGFP, enhanced green fluorescent protein; TGF, transforming growth factor.
Blockade of IL-27 signaling augments reconstitution of Foxp3-expressing Tregs. (A) Balb/c mice were transplanted with B6 Foxp3EGFP BM and spleen cells and then treated with either an isotype control (●) or p28 (□) antibody. Animals were killed 14 days after transplantation and the absolute number of CD4+ and CD8+ Foxp3+ T cells in the spleen, liver, lung, and colon are shown. (B) Sorted CD4+ or CD8+ Foxp3EGFP− T cells were cultured with anti-CD3/28 antibodies, IL-2, and TGF-β alone, or together with graded doses of IL-27. The percentage of CD4+ and CD8+ Foxp3+ T cells is depicted. (C) Balb/c mice were transplanted with B6 Rag-1 BM alone or together with sorted CD4+ (0.4 × 106) and CD8+ (0.2 × 106) Foxp3EGFP− T cells and then treated with either an isotype control (●) or p28 (□) antibody. Animals were killed 14 days posttransplantation and the absolute number of CD4+ and CD8+ Foxp3+ T cells in spleen, liver, lung, and colon is shown. (D) Balb/c mice were transplanted with B6 Foxp3GFP BM and spleen cells and then treated with either an isotype control (▪, n = 10), anti-IL-6R (▲, n = 10), p28 (▼, n = 10), or combination of p28 and anti-IL-6R (♦, n = 10) antibodies. The absolute number of CD4+ and CD8+ Foxp3+ T cells in spleen, liver, lung, and colon 2 weeks posttransplantation is depicted. (E) Balb/c mice transplanted with B6 Rag-1 BM alone (●, n = 5) or with 0.5 × 106 CD4+EGFP− and 0.3 × 106 CD8+EGFP− T cells from Foxp3ΔEGFP animals that had been reconstituted with 40 × 106 to 60 × 106 spleen cells from B6.SJL Foxp3EGFP (CD45.1) animals 1 to 2 days after birth to prevent the development of lethal autoimmunity. Mice transplanted with these T cells were treated with either isotype (▪, n = 10) or p28 antibody (□, n = 10) on days 0 and 6 posttransplantation. Overall survival is depicted. Data are from 2 experiments for each panel. *P < .05, **P < .01, ***P < .001. EGFP, enhanced green fluorescent protein; TGF, transforming growth factor.
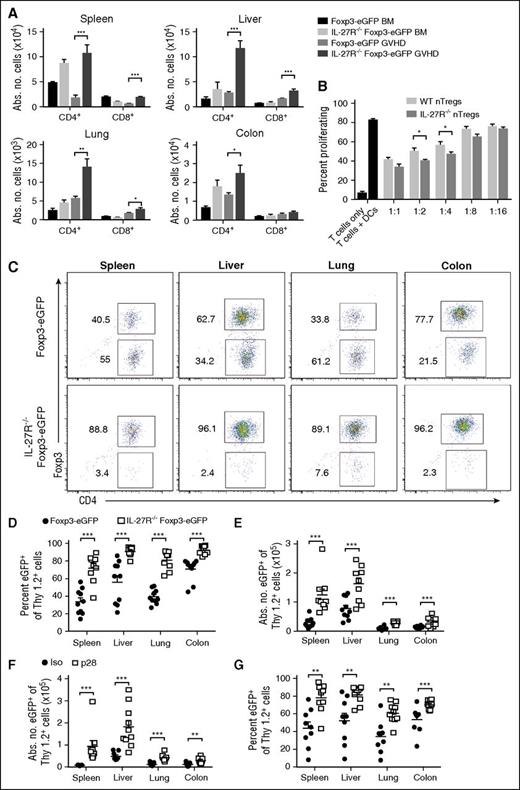
Lack of IL-27R expression of Tregs augments their reconstitution and stabilizes Foxp3 expression posttransplantation
To further assess the effects of IL-27 signaling on Treg reconstitution, we crossed IL-27R−/− and Foxp3EGFP animals to create a reporter mouse so that IL-27R−/− Tregs could be identified in vivo. We observed a significant increase in the absolute number of CD4+ and CD8+ Foxp3+ T cells in mice reconstituted with IL-27R−/−Foxp3EGFP grafts in all GVHD tissues when compared with animals receiving wild-type Foxp3EGFP grafts (Figure 6A), confirming that interruption of IL-27 signaling enhanced Treg reconstitution. To determine the functional competency of these cells, we conducted in vitro assays which demonstrated that CD4+IL-27R−/−Foxp3EGFP T cells were equally suppressive, or even superior at some dilutions, to wild-type CD4+Foxp3EGFP T cells (Figure 6B). To further examine how IL-27R expression affected Treg biology, transplants were conducted in which animals received congenically marked wild-type or IL-27R−/−CD4+ Tregs. We observed that the percentage and absolute number of adoptively transferred Tregs from IL-27R−/−Foxp3EGFP mice that retained Foxp3 expression was significantly higher in all tissue sites when compared with Tregs from wild-type animals (Figure 6C-E). Furthermore, adoptive transfer of CD4+IL-27R−/− Tregs resulted in significantly less GVHD-associated weight loss than wild-type Tregs, although there was no difference in survival (supplemental Figure 8). Finally, transplants were performed in which mice received adoptively transferred CD4+ Tregs from wild-type Foxp3EGFP animals and were then administered an isotype control or p28 antibody. Animals administered p28 antibody had a significantly increased absolute number of CD4+ Tregs (Figure 6F) and there was a higher percentage of Tregs that maintained Foxp3 expression compared with isotype antibody-treated mice (Figure 6G), confirming data observed with congenically marked IL-27R−/−Foxp3EGFPCD4+ T cells.
Absence of IL-27R expression on Tregs enhances reconstitution and stabilizes Foxp3 expression. (A) Lethally irradiated Balb/c recipients were transplanted with B6 Foxp3EGFP BM (black bars, n = 5), IL-27R−/−Foxp3EGFP BM (light gray bars, n = 5), B6 Foxp3EGFP BM and spleen cells (medium gray bars, n = 9), or IL-27R−/−Foxp3EGFP BM and spleen cells (dark gray bars, n = 9) (adjusted to yield an αβ T-cell dose of 0.7 × 106 cells). The absolute number of Tregs in the spleen, liver, lung, and colon is depicted. Data are results from 2 experiments. (B) Column-purified Cell Trace Violet–labeled B6 Thy1.2+ T cells (1 × 105) were cultured with Balb/c CD11c+ dendritic cells (5 × 104) alone or in the presence of varying ratios of sorted CD4+Foxp3EGFP+ or CD4+IL-27R−/−Foxp3EGFP+ Tregs for 5 days in triplicate wells. Control wells are depicted as black bars. Data are presented as the mean percentage ± SEM of Cell Trace Violet low expressing cells and are representative of 1 of 3 experiments with similar results. (C-E) Irradiated Balb/c mice were transplanted with B6.PL BM plus B6.PL spleen cells (0.6 × 106 αβ+ T cells) with either 0.6 × 106 CD4+Foxp3EGFP Tregs or CD4+IL-27R−/−Foxp3EGFP Tregs. Representative dot plots depicting the percentage of Thy1.2+Foxp3+ Tregs in the spleen, liver, lung, and colon that retained expression of Foxp3 2 weeks posttransplantation is shown in panel C. The percentage and absolute number of CD4+Thy1.2+Foxp3+ T cells in the same tissue sites are depicted in panels D and E, respectively. (F-G) Irradiated Balb/c mice were transplanted with B6.PL BM and spleen cells (0.6 × 106 αβ+ T cells) along with 0.6 × 106 sorted Thy1.2+CD4+Foxp3EGFP+ T cells. Cohorts of mice then received either an isotype control or p28 antibody on days 0 and 6. The absolute number and percentage of Thy1.2+CD4+Foxp3+ T cells in the spleen, liver, lung, and colon are depicted in panels F and G. Data are from 2 experiments. *P < .05, **P < .01, ***P < .001.
Absence of IL-27R expression on Tregs enhances reconstitution and stabilizes Foxp3 expression. (A) Lethally irradiated Balb/c recipients were transplanted with B6 Foxp3EGFP BM (black bars, n = 5), IL-27R−/−Foxp3EGFP BM (light gray bars, n = 5), B6 Foxp3EGFP BM and spleen cells (medium gray bars, n = 9), or IL-27R−/−Foxp3EGFP BM and spleen cells (dark gray bars, n = 9) (adjusted to yield an αβ T-cell dose of 0.7 × 106 cells). The absolute number of Tregs in the spleen, liver, lung, and colon is depicted. Data are results from 2 experiments. (B) Column-purified Cell Trace Violet–labeled B6 Thy1.2+ T cells (1 × 105) were cultured with Balb/c CD11c+ dendritic cells (5 × 104) alone or in the presence of varying ratios of sorted CD4+Foxp3EGFP+ or CD4+IL-27R−/−Foxp3EGFP+ Tregs for 5 days in triplicate wells. Control wells are depicted as black bars. Data are presented as the mean percentage ± SEM of Cell Trace Violet low expressing cells and are representative of 1 of 3 experiments with similar results. (C-E) Irradiated Balb/c mice were transplanted with B6.PL BM plus B6.PL spleen cells (0.6 × 106 αβ+ T cells) with either 0.6 × 106 CD4+Foxp3EGFP Tregs or CD4+IL-27R−/−Foxp3EGFP Tregs. Representative dot plots depicting the percentage of Thy1.2+Foxp3+ Tregs in the spleen, liver, lung, and colon that retained expression of Foxp3 2 weeks posttransplantation is shown in panel C. The percentage and absolute number of CD4+Thy1.2+Foxp3+ T cells in the same tissue sites are depicted in panels D and E, respectively. (F-G) Irradiated Balb/c mice were transplanted with B6.PL BM and spleen cells (0.6 × 106 αβ+ T cells) along with 0.6 × 106 sorted Thy1.2+CD4+Foxp3EGFP+ T cells. Cohorts of mice then received either an isotype control or p28 antibody on days 0 and 6. The absolute number and percentage of Thy1.2+CD4+Foxp3+ T cells in the spleen, liver, lung, and colon are depicted in panels F and G. Data are from 2 experiments. *P < .05, **P < .01, ***P < .001.
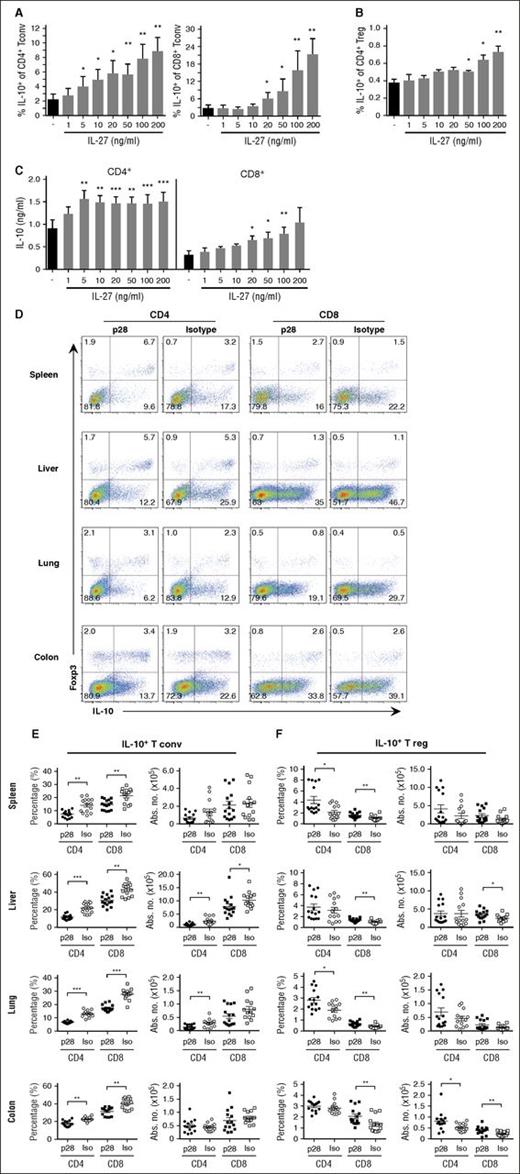
IL-27 signaling blockade has differential effects on IL-10 production by conventional T cells and Tregs
Because IL-10 is one mechanism by which CD4+ Tregs suppress GVHD38 and IL-27 has been shown to enhance T-cell–derived IL-10 secretion in nontransplant models,25,39 we examined whether IL-27 blockade adversely affected IL-10 production by Tregs. To confirm that IL-27 augmented IL-10 production, T cells from IL-10Bit.Foxp3EGFP dual reporter mice were activated with anti-CD3/28 antibodies and IL-2 alone or in the presence of graded doses of IL-27. We observed a dose-dependent increase in the percentage of conventional CD4+ and CD8+ IL-10+ T cells with the effects being most pronounced for CD8+ T cells (Figure 7A). IL-27 also had a statistically significant effect on Treg production of IL-10, but this was biologically negligible (Figure 7B). To confirm that IL-10 detected in these reporter assays was biologically active, we performed enzyme-linked immunosorbent assays which demonstrated elevated levels of IL-10 in supernatants of polyclonally stimulated CD4+ and CD8+ T cells (Figure 7C). To determine whether blockade of IL-27 reduced IL-10 production in vivo, Balb/c recipients were transplanted with marrow grafts from IL-10Bit.Foxp3EGFP reporter animals. Mice administered p28 antibody had a significant reduction in the frequency of conventional CD4+ and CD8+ IL-10+ T cells in all tissue sites (Figure 7D-E). Furthermore, the absolute number of conventional CD4+IL-10+ T cells was reduced in the liver and lung, whereas the number of CD8+IL-10+ T cells was decreased in the liver (Figure 7E). In contrast, there was an increase in the frequency of CD4+ and CD8+ Foxp3+IL-10+ T cells in nearly all tissue sites, as well as an increased absolute number of CD4+ and/or CD8+ IL-10+ Tregs in the colon and liver (Figure 7F). These results indicated that blockade of IL-27 signaling had differential effects on IL-10 production in conventional T cells vs Tregs, and that IL-10 production by Tregs was not adversely affected by inhibition of IL-27.
Antibody blockade of IL-27 has differential effects on IL-10 production in CD4+ and CD8+ conventional T cells vs Tregs. (A-B) Anti-CD3/28 antibody activated CD4+ or CD8+ conventional T cells (Tconv) (A) or Tregs (B) from B6 IL-10 10Bit.Foxp3EGFP reporter mice cultured in IL-2 (30 U/mL) in the absence or presence of graded doses of IL-27 (1-200 ng/mL) for 3 days. The percentage of IL-10–producing conventional or regulatory CD4+ and CD8+ T cells is depicted. (C) IL-10 levels in the culture supernatants of CD4+ and CD8+ T cells stimulated as in panel A as determined by Bioplex assay. Data are derived from 3 experiments. (D-F) Balb/c mice were transplanted with BM and spleen cells from 10Bit.Foxp3EGFP reporter mice and treated with an isotype or p28 antibody on days 0 and 6. Representative dot plots showing the percentage of CD4+ or CD8+ T cells that expressed Foxp3 and IL-10 in the spleen, liver, lung, and colon 10 days posttransplantation (D). The frequency and absolute number of conventional (E) and regulatory (F) CD4+ and CD8+ T cells expressing IL-10 in the spleen, liver, lung, and colon on day 10 are depicted. *P < .05, **P < .01, ***P < .001.
Antibody blockade of IL-27 has differential effects on IL-10 production in CD4+ and CD8+ conventional T cells vs Tregs. (A-B) Anti-CD3/28 antibody activated CD4+ or CD8+ conventional T cells (Tconv) (A) or Tregs (B) from B6 IL-10 10Bit.Foxp3EGFP reporter mice cultured in IL-2 (30 U/mL) in the absence or presence of graded doses of IL-27 (1-200 ng/mL) for 3 days. The percentage of IL-10–producing conventional or regulatory CD4+ and CD8+ T cells is depicted. (C) IL-10 levels in the culture supernatants of CD4+ and CD8+ T cells stimulated as in panel A as determined by Bioplex assay. Data are derived from 3 experiments. (D-F) Balb/c mice were transplanted with BM and spleen cells from 10Bit.Foxp3EGFP reporter mice and treated with an isotype or p28 antibody on days 0 and 6. Representative dot plots showing the percentage of CD4+ or CD8+ T cells that expressed Foxp3 and IL-10 in the spleen, liver, lung, and colon 10 days posttransplantation (D). The frequency and absolute number of conventional (E) and regulatory (F) CD4+ and CD8+ T cells expressing IL-10 in the spleen, liver, lung, and colon on day 10 are depicted. *P < .05, **P < .01, ***P < .001.
Discussion
IL-27 is a member of the IL-12 cytokine family which plays a pivotal role in the biology of GVHD. Two of the most well-studied cytokines in this family, IL-12 and IL-23, have been demonstrated to mediate proinflammatory effects,15,16,40 whereas IL-35 has been reported to have an anti-inflammatory role in GVHD41 as well as other nontransplant models.42,43 The goal of these studies therefore was to define whether IL-27 had a proinflammatory or anti-inflammatory role in GVHD biology. Unexpectedly, as we conducted these studies, we observed discordant results in animals that were reconstituted with marrow grafts from IL-27p28−/− animals vs mice that received IL-27R−/− grafts or were administered a p28-specific antibody with respect to protection from lethal GVHD. Whereas transplantation with IL-27p28−/− grafts exacerbated GVHD, mice reconstituted with IL-27R−/− grafts or those treated with p28 antibody had significantly reduced GVHD. A potential explanation for these results was that T cells from IL-27−/− mice arising in this transgenic environment were more biased toward a proinflammatory phenotype when compared with wild-type T cells. Prior studies by Zhang and colleagues had shown that CD4+ T-cell production of IFN-γ was significantly augmented in mice in which there was conditional deletion of IL-27 from dendritic cells.44 They proposed that the lack of endogenous IL-27 altered T-cell development within the thymus resulting in hyperreactive T cells. Toward that end, we observed that there was a higher percentage of CD8+, as opposed to CD4+, T cells that secreted IFN-γ after polyclonal stimulation or TH1 polarization in vitro, and an increase in the absolute number of CD8+IFN-γ+ T cells in GVHD tissue sites. Moreover, donor CD8+ T cells from IL-27−/− animals induced greater GVHD lethality than wild-type CD8+ T cells when cotransferred with wild-type CD4+ T cells, providing in vivo evidence that the absence of APC-derived IL-27 led to dysregulation of the CD8+ T-cell compartment. Notably, this was not observed in T cells from IL-27R−/− mice where APC production of IL-27 was intact and which induced significantly less GVHD, supporting the p28 antibody results. In fact, both CD4+ and CD8+ IL-27R−/− T cells produced less IFN-γ after TH1 polarization which is consistent with prior studies.23,34,37 Thus, even though CD8+ T cells from both IL-27−/− and IL-27R−/− mice would be similarly primed in vivo, CD8+IL-27−/− T cells had a more exaggerated IFN-γ response after exposure to the GVHD inflammatory milieu which included IL-27 produced by donor BM-derived APCs. Collectively, we believe that the more clinically relevant studies using p28 antibody administration, coupled with the confirmatory results obtained using IL-27R−/− mice, validates that IL-27 plays a proinflammatory role in GVHD biology.
Protection from lethal GVHD by interruption of IL-27 signaling through either antibody-based or genetic approaches was associated with a significant increase in Treg reconstitution in all GVHD target organs. Notably, all Treg populations were increased, including CD8+ Tregs which have also been shown to play a role in GVHD prevention.45 Because IL-27 has direct effects on conventional T cells, it was formally possible that the protective effects were attributable primarily to a reduction in TH1 cells and were not the consequence of augmented Treg reconstitution. To determine whether Treg reconstitution was directly responsible for the reduction in GVHD, we conducted studies in which animals received marrow grafts devoid of all Tregs. Under these conditions, administration of p28 antibody had no protective effects, providing proof that the mechanism by which IL-27 blockade prevented lethal GVHD was by enhancing Treg reconstitution. These results extend the findings of Marillier and colleagues who had previously shown that administration of a p28-specific antibody reduced the severity of GVHD in a nonirradiated parent→F1 model and that this was associated with an increase in the percentage of CD4+ Tregs in secondary lymphoid tissues; but they did not examine whether antibody blockade affected discrete Treg subpopulations, determine whether augmentation of Tregs was evident in target tissue sites, or define whether this was the mechanism by which IL-27 inhibition regulated GVHD.33
Prior studies have demonstrated that CD4+ Tregs express the IL-27R,22 although the functional significance of this has been controversial. Cox and colleagues reported that IL-27 limited Treg conversion in a T-cell transfer model of colitis as well as in an ovalbumin-dependent tolerization model.29 These findings are consistent with previous studies that had shown that IL-27 inhibited the conversion of CD4+Foxp3− into CD4+Foxp3+ T cells both in vitro26,46 and in vivo.29 Conversely, Kim and coworkers noted that transfer of CD4+CD25+ T cells from IL-27R−/− mice induced worse colitis when compared with recipients of wild-type CD4+CD25+ T cells.36 This was associated with reduced Treg numbers in animals reconstituted with IL-27R−/− T cells and the authors concluded that this was due to a requirement for IL-27 to maintain Treg survival. In the current study, we crossed IL-27R−/− mice with Foxp3EGFP animals to create a reporter strain that allowed for the specific detection and selection of CD4+IL-27R−/−Foxp3+ Tregs. These studies revealed that transplantation with marrow grafts from IL-27R−/−Foxp3EGFP mice resulted in a significant increase in the absolute number of CD4+ and CD8+ Tregs in GVHD target tissues. Moreover, the adoptive transfer of Tregs that lacked expression of the IL-27R, or transfer of wild-type Tregs followed by administration of p28 antibody, resulted in a significant increase in the absolute number of these cells in the same tissue sites.
Perhaps the most notable finding was that inhibition of IL-27 signaling resulted in greater stability of Foxp3 expression in CD4+ Tregs. Preclinical studies have shown that the inflammatory milieu arising in the setting of GVHD can lead to loss of Foxp3 expression in both nTreg and iTreg populations.47-49 These so-called ex-Tregs can then acquire the capability to produce TH1-type cytokines, such as IFN-γ, and subsequently contribute to pathological damage.48,49 This raises a legitimate concern that strategies to expand Tregs ex vivo for subsequent adoptive transfer50 may result in untoward inflammatory effects as a consequence of Foxp3 instability. Our observation that antibody blockade of IL-27 not only augmented Treg reconstitution, but also stabilized Foxp3 expression, suggests that this might be a viable clinical approach to promote in vivo Treg reconstitution without the requirement for more costly ex vivo expansion strategies, as well as minimize the potential of Treg conversion to a more pathogenic cellular phenotype.
IL-10 has been shown to be one of the primary mechanisms by which Tregs mitigate the severity of GVHD,38 and absence of donor-derived IL-10 is associated with a significant increase in GVHD-associated mortality.51,52 Thus, IL-10 plays a critical role in the regulation of GVHD. IL-27 has been shown to induce secretion of IL-10 by T cells,25,39 thereby raising the possibility that blockade of IL-27 might deleteriously effect regulation of GVHD by inhibiting this cytokine, even though there was an increase in overall Treg numbers. In fact, we did observe that blockade of IL-27 reduced the frequency of conventional CD4+ and CD8+ IL-10+ T cells in all tissue sites with absolute reductions observed in the liver and lung for CD4+ T cells, and the liver alone for CD8+ T cells. However, antibody-mediated inhibition of IL-27 resulted in an increase in the frequency of CD4+ and CD8+ Foxp3+IL-10+ T cells in nearly all tissue sites, as well as an increased absolute number of CD4+ and/or CD8+ IL-10+ Tregs in the colon and liver. Thus, blockade of IL-27 had differential effects on conventional T cell vs Treg populations, and preserved a critical mechanistic pathway by which Tregs have been shown to suppress GVHD. These results are consistent with our in vitro studies which demonstrated that IL-27 resulted in a significant increase in the percentage of conventional CD4+ and CD8+ T cells that produced IL-10, but had only very modest effects on Tregs, leading one to surmise that blockade of IL-27 would have minimal effects on Treg-induced IL-10 production in vivo which is what we observed. The fact that a reduction in the absolute number of IL-10–producing conventional T cells had no adverse effect on GVHD is consistent with prior studies in which effector T-cell production of IL-10 was shown not to have a regulatory role in mitigating GVHD.51
In summary, we have identified that blockade of the IL-27 signaling pathway resulted in protection from lethal GVHD and this was attributable to enhanced reconstitution of all Treg subsets as well as stabilization of Foxp3 expression. Additionally, inhibition of IL-27 selectively reduced IL-10 production in conventional T cells, but not Tregs, thereby preserving the ability of Tregs to suppress GVHD through this mechanistic pathway. The targeting of IL-27 therefore represents a novel clinical strategy for the in vivo expansion of Tregs and subsequent prevention of GVHD, and provides additional support for the critical role that cytokines in the IL-6 and IL-12 families play in GVHD biology.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from the National Institutes of Health National Heart, Lung, and Blood Institute (HL064603 and HL126166) and by awards from the Midwest Athletes Against Childhood Cancer Fund (W.R.D.). L.B. was supported by a grant from Wallonia Brussels International.
Authorship
Contribution: L.B. performed animal studies and flow cytometric analysis, and helped write the manuscript; K.A. performed research, analyzed data, generated figures, and helped write the manuscript; V.Z., C.Y.-Y., M.G., and R.M. performed research and analyzed data; R.K. performed pathological analysis of all tissue samples; D.E. and B.L. conducted the biostatistical analysis; J.S., D.C., N.G., C.B.W., and J.v.S. provided critical reagents and edited the paper; and W.R.D. developed the overall concept, designed experiments, supervised the study, and wrote the manuscript.
Conflict-of-interest disclosure: N.G. is a present employee of Genentech, a member of the Roche group. D.C. is employed by Merck Research Labs. The remaining authors declare no competing financial interests.
Correspondence: William R. Drobyski, Bone Marrow Transplant Program, Medical College of Wisconsin, 9200 West Wisconsin Ave, Milwaukee, WI 53226; e-mail: wdrobysk@mcw.edu.