Key Points
Platelet transcription of WDR1 suppresses platelet activity.
Reduced transcription of WDR1 in platelets may be a link between elevated platelet activity and increased risk of cardiovascular disease.
Abstract
Platelet activity plays a major role in hemostasis with increased platelet activity likely contributing to the pathogenesis of atherothrombosis. We sought to identify associations between platelet activity variability and platelet-related genes in healthy controls. Transcriptional profiling of platelets revealed that WD-40 repeat domain 1 (WDR1), an enhancer of actin-depolymerizing factor activity, is downregulated in platelet messenger RNA (mRNA) from subjects with a hyperreactive platelet phenotype. We used the human megakaryoblastic cell line MEG-01 as an in vitro model for human megakaryocytes and platelets. Stimulation of MEG-01 with thrombin reduced levels of WDR1 transcripts and protein. WDR1 knockdown (KD) in MEG-01 cells increased adhesion and spreading in both the basal and activated states, increased F-actin content, and increased the basal intracellular calcium concentration. Platelet-like particles (PLPs) produced by WDR1 KD cells were fewer in number but larger than PLPs produced from unmodified MEG-01 cells, and had significantly increased adhesion in the basal state and upon thrombin activation. In contrast, WDR1 overexpression reversed the WDR1 KD phenotype of megakaryocytes and PLPs. To translate the clinical significance of these findings, WDR1 expression was measured in platelet RNA from subjects with established cardiovascular disease (n = 27) and age- and sex-matched controls (n = 10). The WDR1 mRNA and protein level was significantly lower in subjects with cardiovascular disease. These data suggest that WDR1 plays an important role in suppressing platelet activity, where it alters the actin cytoskeleton dynamics, and downregulation of WDR1 may contribute to the platelet-mediated pathogenesis of cardiovascular disease.
Introduction
Platelets are discoid, anucleate cells released by megakaryocytes into the bloodstream where they play a major role in hemostasis, development of atherosclerosis, and pathogenesis of atherothrombosis.1,2 Platelets retain megakaryocyte-derived messenger RNAs (mRNAs) and translational machinery for protein biosynthesis.3-6 Prior clinical studies demonstrated an association between platelet activity and incident cardiovascular morbidity and mortality.7-10 Platelet activity varies greatly among individuals with a small population of healthy subjects noted to have a hyperreactive platelet phenotype.11-14 This variation may, in part, explain some of the variability in the risk for cardiovascular disease (CVD), a platelet-mediated disease.2,15,16 The molecular mechanisms that determine platelet activity variation in the general population are incompletely characterized.
Upon activation with a platelet agonist such as thrombin, intracellular calcium concentration increases, promoting cytoskeletal rearrangements and integrin activation allowing platelet spreading, adhesion, and aggregation.16 Light transmission platelet aggregation in response to submaximal agonist stimulation can reproducibly identify individuals with a heritable hyperreactive platelet phenotype.14,17,18 Subjects with a history of myocardial infarction19,20 and a hyperreactive platelet phenotype12,21 exhibit specific patterns of platelet mRNA expression. Transcriptional profiling of platelets can identify new genes that regulate platelet activity.22,23 For example, myeloid-related protein-14 is more abundant in platelets from patients with acute myocardial infarction19 and increases thrombosis in mice.24 However, the lack of an in vitro model limits the study of potential modulators influencing platelet activity reported in platelet transcription profiling.
In the present study, we performed an unbiased RNA expression profile in platelets of healthy subjects with a hyperactive vs hyporeactive platelet phenotype. WDR1 mRNA expression was significantly lower in subjects with a hyperreactive platelet phenotype. We used the megakaryocytic cell line MEG-01 to model platelet-like activity.25 Thrombin activation of MEG-01 decreased WDR1 mRNA expression and protein levels. Suppression of WDR1 in MEG-01 by short hairpin RNA (shRNA) altered platelet-like functions by increasing megakaryocyte adhesion and spreading, F-actin content, and intracellular calcium concentration. Moreover, platelet-like particles (PLPs) produced from MEG-01 had significantly increased adhesion. Conversely, WDR1 overexpression reverses the phenotype by decreasing adhesion, F-actin content, and attenuating calcium influx and actin cytoskeleton remodeling upon thrombin activation. Finally, levels of platelet WDR1 RNA were significantly lower in subjects with established CVD compared with age- and sex-matched controls. Altogether, our data show that WDR1 suppresses platelet activity and may be an important link between platelet activity and CVD.
Material and methods
Healthy subjects
The studies were conducted in accordance with policies of the New York University Langone Medical Center Institutional Review Board. The discovery cohort consisted of 52 healthy subjects recruited to explore the relationship between platelet activity and platelet-derived mRNA. The validation cohort consisted of a previously described cohort of 50 healthy volunteers who were recruited for a reproducibility study of platelet activity.26 Fifty healthy subjects returned weekly for 3 visits to investigate the reproducibility of platelet activity assays. Methods for inclusion, exclusion, and blood collection are detailed in the supplemental Methods (available on the Blood Web site).
Cardiovascular clinical study
To assess the abundance of platelet WDR1 transcripts in CVD subjects, we identified patients with established vascular disease on aspirin participating in ongoing studies (NCT02106429 and NCT01897103) of platelet activity and platelet transcription profiling in CVD. Exclusion criteria were: use of other antiplatelet therapy including use of anticoagulant and nonsteroidal anti-inflammatory drugs within 72 hours, platelet count <100 or >500, hemoglobin <9, any hemorrhagic diathesis, or severe kidney disease. Age- and sex-matched controls on aspirin monotherapy were selected for platelet mRNA comparison.
Light transmission aggregometry
Light transmission aggregometry was performed within 2 hours of phlebotomy according to the manufacturer’s specification using a Helena AggRAM light transmission aggregometer. As previously described,27 platelet aggregation in response to different agonists at various concentrations was performed: adenosine diphosphate (ADP; 0.4, 1, 2, 4 µM), collagen (0.05, 0.1, 0.2, 1 µg/mL), epinephrine (0.1, 0.4, 2 µM), or arachidonic acid (AA; 30, 150, 1500 µM) and saline (spontaneous platelet aggregation). Percentage of aggregation at 5 minutes and maximum percentage of aggregation was recorded.
Platelet purification
Blood was centrifuged (200g, 10 minutes, room temperature [22°C]) to obtain platelet-rich plasma (PRP). For the discovery cohort, PRP was gel filtered through a Sepharose 2B column (GE Healthcare LifeSciences) and platelets were eluted as described.28 For the validation and CVD cohorts, PRP was centrifuged at 1000g for 10 minutes and the platelet pellet was resuspended in Tyrode buffer. As previously described,29 platelets were subjected to negative selection based on magnetic cell sorting using a human CD45+ and GYPA+ depletion kit (EasySep; Stemcell Technologies). All purified platelets were lysed in QIAzol solution (Qiagen) for RNA extraction following the manufacturer’s instructions for the Direct-zol RNA Miniprep kit (ZymoResearch) and quantified using a Nanodrop ND-2000 spectrophotometer. Leukocyte-depleted platelet (LDP) RNA was used for mRNA studies (supplemental Figure 1). We characterized the purified platelet preparations by real-time polymerase chain reaction (RT-PCR) and flow cytometry. For quantitative PCR analyses, all samples with detection of CD45 transcript prior to 35 cycles of amplication were excluded. The platelet-to-leukocyte ratio was 1 × 107, a purity consistent with other groups measuring platelet RNA.28,30
Gene expression array and quantitative real-time PCR
Total RNA was extracted from LDPs using TRIzol (Invitrogen) and subjected to RNeasy purification (Qiagen) and analyzed using a Bioanalyzer 2100 (Agilent). One hundred nanograms of LDP total RNA was labeled and hybridized to the GeneChip Human Gene U133 Plus 2.0 Array (Affymetrix). The raw intensity values were background corrected, log2 transformed, then normalized using Genespring GX (Agilent). Expression profiles were validated by RT-PCR using gene-specific oligonucleotides (IDT).
Cell culture
MEG-01 cells were purchased from American Type Culture Collection and cultured in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum, 100 U/mL penicillin, and 100 μg/mL streptomycin (Invitrogen) at 37°C in a 5% CO2 humidified atmosphere.
shRNA transfection
TRIPZ-inducible lentiviral shRNA scramble (control) or WDR1 (WDR1 knockdown [KD]) was packaged in the Trans-Lentiviral shRNAPackaging System (Dharmacon) using HEK293T cells for viral production in presence of polyethyleneimine (Sigma-Aldrich). Supernatants were collected 24 hours posttransduction and used to resuspend MEG-01 cells at 2.5 × 105 cells per milliliter in the presence of polybrene overnight. To induce and maintain shRNA production, 1 µg/mL doxycycline was added daily. Fluorescent microscopy, RT-PCR, and immunoblot analysis were used to investigate the efficacy of the shRNA KD. Methods for mRNA quantification, WDR1 overexpression, and immunoblot for WDR1 protein are detailed in the supplemental Methods.
PLP isolation
Spontaneously produced PLPs were isolated from MEG-01 cultures 5 days after the last passage by centrifugation at 200g for 15 minutes. The sediments were discarded and the supernatant was centrifuged at 800g for 15 minutes.31 Supernatants obtained were again centrifuged at 1600g for 15 minutes, and sediments containing PLPs were washed and resuspended in culture medium. To evaluate adhesion, supernatants were incubated with 1 µM DiOC6 (Life Technologies) for 10 minutes at 37°C before the final centrifugation. PLP concentrations were measured by flow cytometry (Accuri C6; BD Biosciences) using CD41 and CD42 antibodies and adjusted to 1 × 104 PLP per microliter for the adhesion assay.
Adhesion
Eighteen-millimeter glass collagen-coated (and noncollaged-coated) coverslips (Helena Laboratories) were blocked with 1% bovine serum albumin in 12-well plates. MEG-01 cells and PLPs were stained for 10 minutes with 1 µM DiOC6, washed, and concentrations were adjusted at 2.5 × 105 cells per milliliter. Cells were transferred in the wells containing the coated or noncoated collagen coverslips in presence or absence of 1 U/mL thrombin (Instrumentation Laboratory) for 12 or 24 hours (activated and no agonist conditions, respectively). After the supernatant was aspirated, adherent cells were gently washed with phosphate-buffered saline. For each well, 5 random fields were captured and area of coverage was quantified using NIH Image J.
Real-time cell electronic sensing test
Cell adhesion and spreading were monitored dynamically using the RTCA DP system (ACEA Bioscience).32
Statistics
Results were reported as mean ± standard error of the mean (SEM) or median ± interquartile range (IQR) where appropriate. The statistical significance between 2 groups was determined by parametric (Student t test) or nonparametric (Mann-Whitney U test) testing, as appropriate. P < .05 was considered statistically significant.
Methods for in vitro cell spreading, F:G-actin measurements, phalloidin staining, and calcium mobilization are detailed in the supplemental Methods.
Results
Platelets from healthy donors divided into hyperreactive and hyporeactive groups
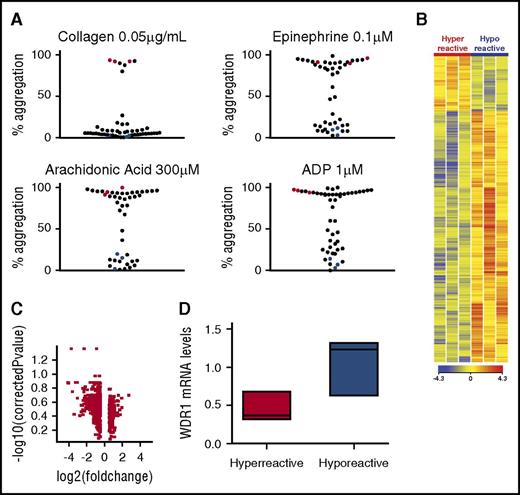
To identify patients with differential platelet reactivity, 52 healthy subjects were recruited for platelet phenotype analysis (supplemental Table 1). Data from our group and others demonstrate that use of submaximal concentrations of agonists can identify a population with a hyperreactive platelet phenotype.11,14 To identify extremes in the healthy population, subjects were categorized as having a hyperreactive platelet phenotype if the percentage of aggregation was >80% to submaximal concentrations across different agonists, including ADP (1 μM), collagen (0.05 μg/mL), epinephrine (0.1 μM), and AA (300 μM). A comparison group of subjects with a hyporeactive platelet phenotype was considered if the percentage of aggregation was <20% to each agonist. Figure 1A shows the bimodal platelet aggregation response to submaximal agonist concentrations for 52 healthy subjects. Six age- and sex-matched subjects fulfilled these criteria and were used for the platelet RNA expression study. The maximum aggregation response was significantly different between the hyperreactive and hyporeactive groups (P < .001 for each comparison, n = 3 for each group). Platelet count and mean platelet volume were similar between groups (supplemental Table 1).
Platelet mRNA profiling in 6 healthy subjects and downregulation of WDR1 expression in participants with hyperreactive vs hyporeactive participants. (A) Platelet aggregation measurements by aggregometry in response to low doses of platelet agonists. Each dot represents the percentage of aggregation for each individual subject in response to submaximal collagen (0.05 µg/mL), epinephrine (0.1 µM), AA (300 µM), or ADP (1 µM). Subjects with hyperreactive platelets have >80% aggregation whereas subjects with hyporeactive platelets have <20% aggregation in response to collagen, epinephrine, AA, and ADP. Red and blue dots represent the 6 subjects with hyperreactive (n = 3) and hyporeactive (n = 3) platelet phenotypes, respectively. The maximum aggregation response was used to classify groups and was thus significantly different between the 3 hyperreactive and 3 hyporeactive subjects (ADP, 94.5% ± 2.7% vs 11.3% ± 3.7%; collagen, 90.9% ± 2.6% vs 5.5% ± 3.8%; epinephrine, 92.7% ± 2.9% vs 8.0% ± 4.4%; AA, 91.1% ± 3.6% vs 9.0% ± 6.9%; mean ± SEM). (B) Heatmap of platelet mRNA gene expression in subjects with a hyperreactive vs a hyporeactive platelet phenotype. Red and blue characterize upregulated and downregulated genes, respectively. (C) Volcano plot for genes with a fold change higher than 1.5 between hyperreactive and hyporeactive platelets. (D) Validation of WDR1 expression by quantitative PCR in subjects with a hyperreactive (n = 3) vs a hyporeactive (n = 3) platelet phenotype (P = .10, 1-sided Mann-Whitney U test). Data are median ± IQR. *P < .05, hyperreactive vs hyporeactive group.
Platelet mRNA profiling in 6 healthy subjects and downregulation of WDR1 expression in participants with hyperreactive vs hyporeactive participants. (A) Platelet aggregation measurements by aggregometry in response to low doses of platelet agonists. Each dot represents the percentage of aggregation for each individual subject in response to submaximal collagen (0.05 µg/mL), epinephrine (0.1 µM), AA (300 µM), or ADP (1 µM). Subjects with hyperreactive platelets have >80% aggregation whereas subjects with hyporeactive platelets have <20% aggregation in response to collagen, epinephrine, AA, and ADP. Red and blue dots represent the 6 subjects with hyperreactive (n = 3) and hyporeactive (n = 3) platelet phenotypes, respectively. The maximum aggregation response was used to classify groups and was thus significantly different between the 3 hyperreactive and 3 hyporeactive subjects (ADP, 94.5% ± 2.7% vs 11.3% ± 3.7%; collagen, 90.9% ± 2.6% vs 5.5% ± 3.8%; epinephrine, 92.7% ± 2.9% vs 8.0% ± 4.4%; AA, 91.1% ± 3.6% vs 9.0% ± 6.9%; mean ± SEM). (B) Heatmap of platelet mRNA gene expression in subjects with a hyperreactive vs a hyporeactive platelet phenotype. Red and blue characterize upregulated and downregulated genes, respectively. (C) Volcano plot for genes with a fold change higher than 1.5 between hyperreactive and hyporeactive platelets. (D) Validation of WDR1 expression by quantitative PCR in subjects with a hyperreactive (n = 3) vs a hyporeactive (n = 3) platelet phenotype (P = .10, 1-sided Mann-Whitney U test). Data are median ± IQR. *P < .05, hyperreactive vs hyporeactive group.
WDR1 is downregulated in hyperreactive platelets
Previous studies have shown that platelet transcriptional profiling can offer insight into the pathogenesis of platelet-mediated diseases.12 To identify new genes associated with platelet function, we performed an unbiased mRNA expression profile by platelet phenotype. As illustrated in Figure 1B, distinct patterns of gene expression were present between subjects with a hyperreactive vs hyporeactive platelet phenotype. Using a nominal P value of .05 and a fold-change higher than 2.0, we found 115 transcripts that were differentially expressed between the groups (Figure 1C). Pathway analysis using the Gene Ontology bioinformatics annotation resources indicated that many genes related to protein and vesicular transport and actin cytoskeleton were downregulated in the hyperreactive group (supplemental Table 2). Cross-comparing our candidate gene list to the list of genes involved in hemostasis generated by Reactome,33 we found that 17 of our mRNA candidates were downregulated in hyperreactive platelets, compared with hyporeactive platelets, and linked to hemostasis (supplemental Table 3). Although at least 12 of the 17 genes have been characterized as regulators of platelet function, WDR1 was of particular interest because its involvement in megakaryocyte and platelet development has been described,34,35 yet its role in megakaryocyte or platelet function and subsequent risk of CVD is unknown.
We then confirmed the expression profile findings for WDR1 expression in platelets by RT-PCR. Median WDR1 mRNA expression was 70% lower in the hyperreactive platelets than in the hyporeactive platelets (P = .10; Figure 1D). Because our preliminary observation was made from 6 individuals, we sought to test the robustness of our findings in a separate cohort of healthy subjects (validation cohort). Among 50 healthy subjects recruited into a platelet activity reproducibility study,26 we identified 10 subjects with a consistent platelet phenotype and platelet RNA available (6 hyporeactive and 4 hyperreactive platelet phenotypes; supplemental Figure 2). Median WDR1 mRNA expression was 45% lower in the hyperreactive platelets compared with the control platelets (P = .0055; supplemental Figure 3; supplemental Table 1). To confirm the association between WDR1 and platelet activity, we investigated WDR1 mRNA in all 50 subjects from the validation cohort. A summary platelet score was used that combined 17 different measurements of platelet activity (light transmission aggregation and impedance aggregation in response to many different agonists).36 We observed a statistically significant negative correlation between platelet WDR1 mRNA expression levels and platelet score in the 50 subjects of this validation cohort (r = −0.33; P = .0460; supplemental Figure 4). Similar associations were observed between WDR1 and platelet aggregation in response to submaximal epinephrine, ADP, collagen and arachidonic acid (data not shown).
Activation of the megakaryocyte cell line, MEG-01, leads to WDR1 downregulation
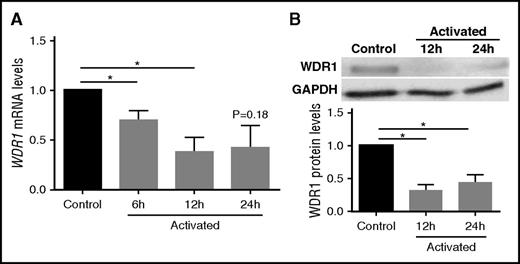
We investigated whether the expression of WDR1 is detectable in the megakaryoblastic cell line MEG-01, which exhibits similar properties to platelets and is frequently used as a model for human megakaryocytes and a source of platelet precursors.25,37,38 Because the hyperreactive platelet phenotype displayed reduced expression of WDR1, we hypothesized that activated MEG-01 cells, as platelet precursors, would reduce expression of WDR1 mRNA, similar to the platelet mRNA from subjects with a hyperreactive platelet phenotype. Indeed, when we stimulated MEG-01 cells with thrombin on collagen-coated coverslips, we observed a 60% decrease in WDR1 transcript levels (P = .03; Figure 2A) and a 66% decrease in WDR1 protein levels (P < .0001; Figure 2B) at 12 hours postactivation compared with control MEG-01 cells (absence of thrombin and noncoated coverslips). The reduced WDR1 expression following megakaryocyte activation is consistent with the downregulation of WDR1 in the hyperreactive vs hyporeactive platelet phenotype, and suggests a role for WDR1 in suppressing the platelet-like-properties of megakaryocytes.
WDR1 is downregulated in activated MEG-01 cells. (A) WDR1 gene expression in MEG-01 cells was measured at baseline and after treatment with thrombin 1 U/L on collagen-coated coverslips at 6, 12, and 24 hours. (P = .04, 0.03, and 0.18, respectively, Student t test with Bonferroni correction). (B) Representative immunoblots probed for WDR1 in MEG-01 cells and quantification of the decrease in WDR1 protein after treatment with thrombin and collagen at 12 and 24 hours (P = .004 and <.0001, respectively, Student t test with Bonferroni correction). Activated MEG-01 cells were compared with control MEG-01 cells (absence of thrombin and noncoated coverslips). Data are mean ± SEM of 6 experiments. *P < .05, vs unstimulated (no thrombin or collagen) control MEG-01 cells. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
WDR1 is downregulated in activated MEG-01 cells. (A) WDR1 gene expression in MEG-01 cells was measured at baseline and after treatment with thrombin 1 U/L on collagen-coated coverslips at 6, 12, and 24 hours. (P = .04, 0.03, and 0.18, respectively, Student t test with Bonferroni correction). (B) Representative immunoblots probed for WDR1 in MEG-01 cells and quantification of the decrease in WDR1 protein after treatment with thrombin and collagen at 12 and 24 hours (P = .004 and <.0001, respectively, Student t test with Bonferroni correction). Activated MEG-01 cells were compared with control MEG-01 cells (absence of thrombin and noncoated coverslips). Data are mean ± SEM of 6 experiments. *P < .05, vs unstimulated (no thrombin or collagen) control MEG-01 cells. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
WDR1 represses MEG-01 adhesion and spreading
To investigate whether the downregulation of WDR1 had any functional effect on megakaryocyte and PLP activity, we transduced MEG-01 cells with lentivirus expressing either WDR1-specific or scrambled shRNA to generate WDR1 KD and their control cells. WDR1 mRNA and protein levels decreased by 79% (P < .0001) and 39% (P = .0041), respectively (Figure 3A-B). Similar to megakaryocytes from the WDR1 KD in mice,34 WDR1 KD cells were smaller than controls (supplemental Figure 5).
WDR1 knockdown in MEG-01 cells affects cell size, adhesion, and spreading. MEG-01 cells were transfected with scrambled shRNA (control) or WDR1 shRNA (WDR1 KD). (A) WDR1 mRNA and protein expression in WDR1 KD and control MEG-01 cells. Data are mean ± SEM for 3 independent experiments. (B) WDR1 protein expression in WDR1 KD and control MEG-01, in the absence of agonist with noncoated glass coverslips or stimulated for 12 hours with thrombin 1 U/mL and adherent on collagen-coated coverslips and quantification. Data are mean ± SEM for 3 independent experiments (P = .004, .005, and .002 respectively, Student t test). (C) Adhesion of WDR1 KD and control MEG-01 cells (green) in the absence and presence of thrombin 1 U/mL on collagen-coated coverslips and adhesion quantification (P < .0001 and P = .0002, respectively, Student t test) Data are mean ± SEM for 3 independent experiments. (D) Assay for adhesion and spreading of WDR1 KD and control MEG-01 cells following stimulation with thrombin 1 U/mL and collagen 5 µg/mL using the xCELLigence system. The xCELLigence system measured impedance every 4 minutes for 24 hours, reported as a cell index value. Lines and bars = mean ± SEM of quadruplicates. Similar results were obtained in an additional 2 experiments. *P < .05 vs unstimulated control MEG-01 cells. AU, arbitrary unit.
WDR1 knockdown in MEG-01 cells affects cell size, adhesion, and spreading. MEG-01 cells were transfected with scrambled shRNA (control) or WDR1 shRNA (WDR1 KD). (A) WDR1 mRNA and protein expression in WDR1 KD and control MEG-01 cells. Data are mean ± SEM for 3 independent experiments. (B) WDR1 protein expression in WDR1 KD and control MEG-01, in the absence of agonist with noncoated glass coverslips or stimulated for 12 hours with thrombin 1 U/mL and adherent on collagen-coated coverslips and quantification. Data are mean ± SEM for 3 independent experiments (P = .004, .005, and .002 respectively, Student t test). (C) Adhesion of WDR1 KD and control MEG-01 cells (green) in the absence and presence of thrombin 1 U/mL on collagen-coated coverslips and adhesion quantification (P < .0001 and P = .0002, respectively, Student t test) Data are mean ± SEM for 3 independent experiments. (D) Assay for adhesion and spreading of WDR1 KD and control MEG-01 cells following stimulation with thrombin 1 U/mL and collagen 5 µg/mL using the xCELLigence system. The xCELLigence system measured impedance every 4 minutes for 24 hours, reported as a cell index value. Lines and bars = mean ± SEM of quadruplicates. Similar results were obtained in an additional 2 experiments. *P < .05 vs unstimulated control MEG-01 cells. AU, arbitrary unit.
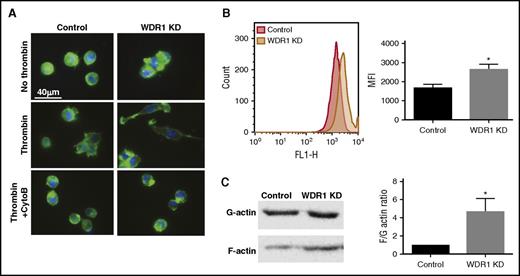
Upon thrombin and collagen activation, megakaryocytes and platelets undergo cytoskeletal remodeling which promotes adhesion, aggregation, and the formation of filopodia and lamellipodia.39-42 Because these processes are mediated by actin and WDR1 plays a major role in actin depolymerization,43 we investigated whether WDR1 KD impairs these processes. We measured adhesion to collagen-coated coverslips in the basal and activated states. Representative micrographs (Figure 3C) show increased adhesion of WDR1 KD megakaryocytes in the basal condition (20.6% ± 17.8% vs 2.2% ± 1.5%, P < .0001) and following thrombin activation (66.6% ± 17.5% vs 49.7% ± 14.8%, P = .0002) compared with control MEG-01 cells. Similar results were obtained with MEG-01 cells transfected with WDR1 siRNA (data not shown). Using impedance technology to follow adhesion and spreading in a real-time manner, we observed that maximal adhesion is higher in the WDR1 KD vs control that extended 24 hours (Figure 3D). Conversely, after lentiviral infection of MEG-01 to induce constitutive WDR1 overexpression (supplemental Figure 6A), adhesion was significantly reduced in WDR1-overexpressing MEG-01 cells with or without thrombin activation (supplemental Figure 6B). These data suggest that megakaryocyte adhesion and spreading is enhanced in the WDR1 KD cells, and is reversed in the WDR1-overexpressing cells.
WDR1 represses actin remodeling and cell spreading
WDR1 is a partner of cofilin, an actin-binding protein that has a central role in controlling actin dynamics.44 We hypothesized that WDR1 KD would decrease actin depolymerization, thereby impacting remodeling of the cytoskeleton and increasing the formation of actin stress fibers, pseudopodia, and lamellipodia. These cytoskeletal dynamics depend on actin filament (F-actin) growth from the polymerization of G-actin monomers.42 To observe cytoskeletal changes upon thrombin activation, we stained nonactivated and activated cells with phalloidin, which binds to polymerized F-actin. In nonactivated cells, cytoplasmic F-actin accumulation and large aggregates were observed in the WDR1 KD cells (Figure 4A left panel). Upon thrombin activation, WDR1 KD cells were more spread, with more extensions and phalloidin staining vs controls. As expected, treatment of WDR1 KD megakaryocytes with cytochalasin B, a fungal metabolite that inhibits actin assembly, limited megakaryocyte adhesion and spreading (Figure 4A right panel). On the contrary, in WDR1-overexpressing cells, F-actin content was reduced and megakaryocytes lost spreading ability and conserved their round shape (supplemental Figure 6C). Using flow cytometry to measure phalloidin staining, we observed a higher F-actin content in the WDR1 KD vs control MEG-01 cells (Figure 4B). Furthermore, the F-to-G actin ratio was significantly increased in the WDR1 KD cells compared with control cells (Figure 4C). These data suggest that WDR1 regulation alters actin cytoskeleton dynamics where WDR1 KD increases F-actin and promotes spontaneous activation and adhesion, WDR1 overexpression produces the opposite phenotype.
WDR1 regulates actin cytoskeleton dynamics. (A) F-actin staining with phalloidin of adherent WDR1 KD or control cells 12 hours after stimulation with or without thrombin 1 U/mL on collagen-coated coverslips. In the bottom right panel, WDR1 KD MEG-01 cells were also treated with cytochalasin B, an inhibitor of actin polymerization. F-actin (green), 4′,6-diamidino-2-phenylindole (blue). (B) Unactivated WDR1 KD or control MEG-01 cells stained with phalloidin-fluorescein isothiocyanate were analyzed on flow cytometry. Data are mean ± SEM of 3 experiments (P = .02, Student t test). (C) Comparison of F-actin-to-G-actin ratio in control and WDR1 KD MEG-01 (left panel) based on a immunoblot analysis (right panel). Data are mean ± SEM of 3 experiments (P = .04, Student t test). *P < .05 vs control MEG-01 cells. MFI, mean fluorescence intensity.
WDR1 regulates actin cytoskeleton dynamics. (A) F-actin staining with phalloidin of adherent WDR1 KD or control cells 12 hours after stimulation with or without thrombin 1 U/mL on collagen-coated coverslips. In the bottom right panel, WDR1 KD MEG-01 cells were also treated with cytochalasin B, an inhibitor of actin polymerization. F-actin (green), 4′,6-diamidino-2-phenylindole (blue). (B) Unactivated WDR1 KD or control MEG-01 cells stained with phalloidin-fluorescein isothiocyanate were analyzed on flow cytometry. Data are mean ± SEM of 3 experiments (P = .02, Student t test). (C) Comparison of F-actin-to-G-actin ratio in control and WDR1 KD MEG-01 (left panel) based on a immunoblot analysis (right panel). Data are mean ± SEM of 3 experiments (P = .04, Student t test). *P < .05 vs control MEG-01 cells. MFI, mean fluorescence intensity.
WDR1 represses platelet-like particle activity
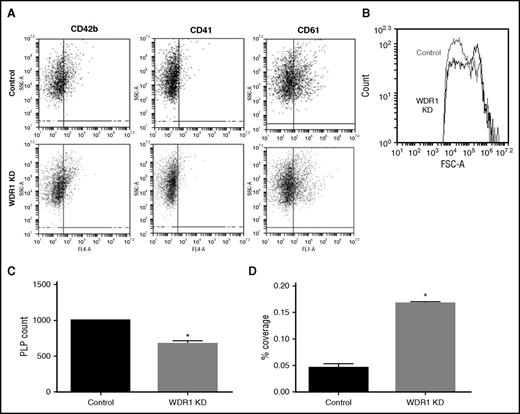
Given that MEG-01 cells spontaneously produce PLPs with activity and functionality similar to human platelets,25 we tested the effect of WDR1 expression on PLP production and activity. To evaluate PLPs produced by MEG-01 cells, we analyzed CD41 (GPIIb), CD42b (GPIb), and CD61 (GPIIIa) positive events with similar size and complexity vs peripheral blood platelets (supplemental Figure 7). Levels of CD41, CD42b, and CD61 were similar between PLPs produced from WDR1 KD and control MEG-01 cells (Figure 5A). Consistent with data observed from the mouse WDR1 KD,34 PLPs were larger as reflected by increased forward side scatter (Figure 5B) and significantly fewer (Figure 5C) in the WDR1 KD than controls. To evaluate PLP activity, we performed an adhesion assay. After adjustment of PLP concentrations, adhesion of WDR1 KD PLPs was increased compared with control PLPs (P = .003; Figure 5D). Taken together, these results demonstrate that PLPs produced from the MEG-01 WDR1 KD are larger with greater adhesion.
WDR1 knockdown increases PLP size and adhesion but decreases PLP production. (A) Expression of CD42b, CD41, and CD61 platelet markers at the PLP surface analyzed by flow cytometry in WDR1 KD and control PLPs. (B) Forward side scatter (FSC) of PLPs produced by WDR1 KD and control MEG-01 cells. WDR1 KD-derived PLPs are larger than PLPs derived from controls. Similar results were obtained in an additional 2 experiments. (C) Quantification of PLP production by WDR1 KD and control MEG-01 cells (P = .02, Student t test). (D) Quantification of PLP adhesion upon stimulation with collagen and thrombin. WDR1 KD-derived PLPs are more than threefold more adherent than their control (P = .003, Student t test). Data are mean ± SEM of 3 experiments. *P < .05 vs control MEG-01-derived PLPs. SSC-A, side scatter.
WDR1 knockdown increases PLP size and adhesion but decreases PLP production. (A) Expression of CD42b, CD41, and CD61 platelet markers at the PLP surface analyzed by flow cytometry in WDR1 KD and control PLPs. (B) Forward side scatter (FSC) of PLPs produced by WDR1 KD and control MEG-01 cells. WDR1 KD-derived PLPs are larger than PLPs derived from controls. Similar results were obtained in an additional 2 experiments. (C) Quantification of PLP production by WDR1 KD and control MEG-01 cells (P = .02, Student t test). (D) Quantification of PLP adhesion upon stimulation with collagen and thrombin. WDR1 KD-derived PLPs are more than threefold more adherent than their control (P = .003, Student t test). Data are mean ± SEM of 3 experiments. *P < .05 vs control MEG-01-derived PLPs. SSC-A, side scatter.
Calcium influx modulation by WDR1
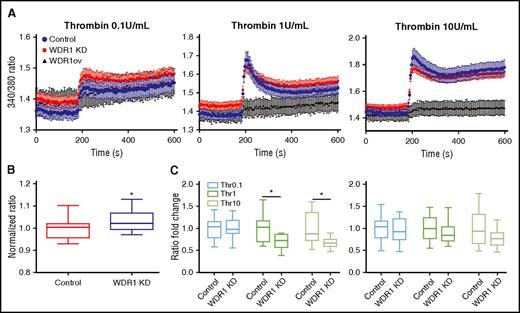
Intracellular calcium concentration and store-operated calcium entry are tightly regulated by actin cytoskeleton dynamics.45 Upon stimulation, platelets rapidly increase intracellular calcium concentrations after actin cytoskeleton reorganization enables influx leading to platelet activation and shape change.39,42,45 Similar to calcium influx in platelets, we showed that immediately after addition of thrombin, cytosolic calcium concentration increased in MEG-01 cells, peaked, then plateaued (Figure 6A). The baseline concentration of calcium was higher in WDR1 KD cells than MEG-01 controls (Figure 6B). There was no difference in calcium influx in the presence of 1 U/mL thrombin. Using supraphysiologic doses of thrombin, calcium influx characterized by the difference between baseline and peak was lower in WDR1 KD cells than MEG-01 controls (Figure 6C left panel). No significant difference in cytosolic calcium concentrations was observed in the plateau phase (Figure 6C right panel). Conversely, WDR1-overexpressing cells did not respond to thrombin stimulation. These data suggest a preactivated state of the WDR1 KD cells.
WDR1 affects calcium influx. (A) Calcium concentration measured over time before and after addition of thrombin (0.1 U/mL, 1 U/mL, and 10 U/mL) at 180 seconds in MEG-01 cells. (B) Baseline calcium concentration was higher in WDR1 KD cells than controls (P < .0001, Student t test). (C) Quantification of calcium concentration change between baseline and peak calcium influx (left panel) (P = .0004 and P = .0001, respectively, Student t test); and between baseline and plateau levels, after 300 seconds (right panel). Data are median ± IQR of 10 experiments. *P < .05 vs control MEG-01 cells.
WDR1 affects calcium influx. (A) Calcium concentration measured over time before and after addition of thrombin (0.1 U/mL, 1 U/mL, and 10 U/mL) at 180 seconds in MEG-01 cells. (B) Baseline calcium concentration was higher in WDR1 KD cells than controls (P < .0001, Student t test). (C) Quantification of calcium concentration change between baseline and peak calcium influx (left panel) (P = .0004 and P = .0001, respectively, Student t test); and between baseline and plateau levels, after 300 seconds (right panel). Data are median ± IQR of 10 experiments. *P < .05 vs control MEG-01 cells.
WDR1 is downregulated in patients with cardiovascular diseases
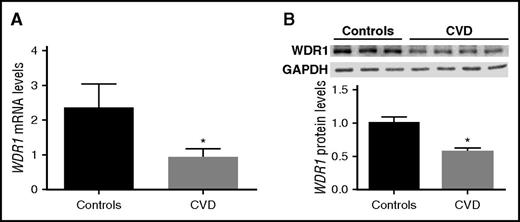
As shown in Figure 1, subjects with a hyperreactive vs hyporeactive platelet phenotype have significantly lower WDR1 expression. We also showed that WDR1 KD increases megakaryocyte and platelet-like activities. We therefore hypothesized that subjects with CVD (a disease process mediated by platelet activity)15 would have reduced WDR1 expression and protein production in platelets. To test our hypothesis, 37 subjects (27 subjects with CVD and 10 controls that were age-, sex- and race-matched) were evaluated. As expected, platelet activity was higher in subjects with CVD than controls (data not shown). Gene expression analysis for WDR1 in the 2 groups showed a significantly lower level of platelet-derived WDR1 mRNA levels in subjects with CVD vs matched controls (P = .0148; Figure 7A). Western blot analysis confirmed the downregulation of WDR1 in subjects with established CVD vs controls (P = .0078; Figure 7B).
WDR1 expression is reduced in subjects with cardiovascular diseases. (A) Platelet-derived WDR1 mRNA levels were measured in platelets from 10 healthy subjects and 27 age-, race-, and sex-matched subjects with CVD (P = .01, Student t test). (B) WDR1 protein expression in 4 CVD subjects vs 3 healthy controls (P = .008, Student t test). Data are mean ± SEM. *P < .05 vs control subjects.
WDR1 expression is reduced in subjects with cardiovascular diseases. (A) Platelet-derived WDR1 mRNA levels were measured in platelets from 10 healthy subjects and 27 age-, race-, and sex-matched subjects with CVD (P = .01, Student t test). (B) WDR1 protein expression in 4 CVD subjects vs 3 healthy controls (P = .008, Student t test). Data are mean ± SEM. *P < .05 vs control subjects.
Discussion
Human platelets from healthy individuals show remarkable interindividual variation in response to submaximal agonists, with aggregation varying from 1% to 100% (Figure 1A). Mechanisms underlying this observation remain poorly understood. Platelets are anucleated cells released in the circulation by fragmentation of megakaryocyte progenitors.46 During this process, mRNA and functional translational machinery for de novo protein synthesis are packaged into the platelet cytoplasm.4,5,47 Using transcriptional profiling of platelets with different levels of reactivity, we identified a set of genes whose expression differed significantly between individuals with hyperreactive and hyporeactive platelet phenotypes. Our major finding was that the WDR1 transcript was approximately equal to twofold lower in platelets from subjects with a hyperreactive vs hyporeactive platelet phenotype in our discovery and validation cohorts. This is consistent with earlier published reports demonstrating reduced levels of WDR1 protein in platelets from smokers and WDR1 platelet mRNA in patients with chronic kidney disease, both known to have increased platelet reactivity.29,48 Although studies of WDR1-deficient mice showed that WDR1 is required for megakaryopoiesis and platelet formation,34 the role of WDR1 in platelet function has not been investigated.
Because platelets are anucleated cells viable for only a few hours outside the body, the lack of in vitro models to investigate the role of specific genes in platelet activity is an important limitation for validation of certain genes. To overcome this, we developed a platelet-like activity assay using the MEG-01 megakaryocytic cell line. Exposure of MEG-01 cells to platelet agonists increases their adherence and aggregation.25,49 These changes are accompanied by calcium influx into the cytoplasm, triggering reorganization of the actin cytoskeleton.25 Based on these platelet-like properties, we hypothesized thrombin-activated MEG-01 cells could alter transcription and translation of WDR1. In fact, WDR1 expression and protein production was reduced in thrombin-activated MEG-01 cells, similar to our observations in platelets from subjects with a hyperreactive phenotype.
WDR1 protein is a major cofactor of actin depolymerizing factor (ADF)/cofilin.50 WDR1 can bind to the ADF-cofilin-F-actin complex and strongly enhance the severing activity of ADF-cofilin on actin filaments resulting in acceleration of actin disassembly. Considering its major role in dynamics of the actin cytoskeleton, we hypothesized that WDR1 downregulation affects shape, size, and function of MEG-01 cells and their derived PLPs.
Although murine WDR1 knockout is embryonically lethal,34 WDR1 KD induces a spontaneous inflammatory disease and macrothrombocytopenia. The lack of cofilin abolishes depolymerization of actin and actin filament severing whereas the lack of WDR1 reduces it.43 Compromised actin dynamics reduces proplatelet formation and platelet shedding, and reduces rate of depolymerization, causing decreased fragmentation rate, increasing platelet size without affecting platelet clearance rate, as shown in WDR1 KD mice. Compared with smaller platelets, larger platelets are metabolically and enzymatically more active, have greater prothrombotic potential, and associate with a heightened risk of cardiovascular events.26,51,52 Indeed, in our WDR1 KD model, we observed that MEG-01 cells were smaller (supplemental Figure 5) and produced larger PLP, similar to the observation for KD of the mouse homolog of WDR1.34
Adhesion is an essential platelet function controlled by adhesive ligands and receptor pairs and modulation of the actin cytoskeleton.53 Our functional studies for both megakaryocytes and PLPs demonstrated enhanced adhesion for the WDR1 KD in both basal and thrombin-activated states. Decreasing actin depolymerization by knocking down WDR1 expression in MEG-01 cells promotes actin cytoskeleton disorganization and increases the relative abundance of F-actin and presence of actin aggregates, consistent with previous findings that depletion of WDR1 or its homolog in various organisms leads to abnormal actin accumulation.54-56 Furthermore, inhibition of actin depolymerization in cells lacking WDR1 also increases intracellular calcium concentrations at baseline, further promoting actin cytoskeletal rearrangements which in turn can lead to enhanced adhesion to collagen.57,58 Collectively, our data suggest that diminishing actin depolymerization via WDR1 KD promotes the stabilization of F-actin and contributes to the activation and binding to the collagen matrix of WDR1 KD cells, thereby increasing platelet adhesion.
Finally, our cohort of patients with CVD had lower levels of platelet WDR1 mRNAs and protein than their matched counterparts without CVD. The downregulation of WDR1 in 2 independent cohorts of subjects with hyperreactive platelets, in a cohort of patients with CVD, emphasizes the unique role of WDR1 in platelet function and in patients with established CVD.
Taken together, these data suggest WDR1 is a key regulator of platelet activity and CVD that alters actin cytoskeleton dynamics. This bedside-to-bench approach toward measuring the platelet transcriptome in well-phenotyped populations suggests other molecular candidates identified as potential regulators of platelet reactivity can be validated using an in vitro megakaryocytic cell line model that mimics platelet function. Because increased platelet activity is associated with cardiovascular morbidity and mortality, describing the role of other candidate genes associated with increased platelet activity could identify new modulators of platelet activity and help establish potential diagnostic and therapeutic targets.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors are grateful to Coen Van Solingen for help with cloning and Stefan Feske for advice and expertise with the calcium mobilization assays.
This work was supported in part by the National Heart, Lung, and Blood Institute of the National Institutes of Health (R01HL114978) (J.S.B.), the American Heart Association Clinical Research Program (13CRP14410042) (J.S.B.), and the Doris Duke Clinical Scientist Development Award (2010055) (J.S.B.).
E.A. was supported by a Howard Hughes Medical Institute International Student Research Fellowship.
Authorship
Contribution: E.M. designed and performed the research, analyzed the results, and wrote the manuscript; C.E., N.A., and E.A. performed experiments; and Y.S. and J.S.B. designed the research, analyzed the results, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jeffrey S. Berger, Marc and Ruti Bell Program in Vascular Biology, Cardiovascular Clinical Research Center, New York University School of Medicine, 530 First Ave, Skirball 9R, New York, NY 10016; e-mail: jeffrey.berger@nyumc.org.