Key Points
Aged neutrophils exhibit a distinct, highly reactive phenotype that depends on age-related changes in their molecular repertoire.
This specific phenotype of aged neutrophils enables them to serve as “first responders” in inflammatory reactions.
Abstract
Under steady-state conditions, aged neutrophils are removed from the circulation in bone marrow, liver, and spleen, thereby maintaining myeloid cell homeostasis. The fate of these aged immune cells under inflammatory conditions, however, remains largely obscure. Here, we demonstrate that in the acute inflammatory response during endotoxemia, aged neutrophils cease returning to the bone marrow and instead rapidly migrate to the site of inflammation. Having arrived in inflamed tissue, aged neutrophils were found to exhibit a higher phagocytic activity as compared with the subsequently recruited nonaged neutrophils. This distinct behavior of aged neutrophils under inflammatory conditions is dependent on specific age-related changes in their molecular repertoire that enable these “experienced” immune cells to instantly translate inflammatory signals into immune responses. In particular, aged neutrophils engage Toll-like receptor-4- and p38 MAPK-dependent pathways to induce conformational changes in β2 integrins that allow these phagocytes to effectively accomplish their mission in the front line of the inflammatory response. Hence, ageing in the circulation might represent a critical process for neutrophils that enables these immune cells to properly unfold their functional properties for host defense.
Introduction
Leukocyte recruitment from the microvasculature to the site of inflammation is a fundamental process in the inflammatory response. Infiltrating neutrophilic granulocytes (neutrophils) constitute the first line of defense against invading pathogens, followed by a second wave of inflammatory monocytes enforcing the inflammatory reaction.1-3
Neutrophils represent the predominant myeloid leukocyte subset in most mammals.1,2 The life span of these phagocytes is thought to be relatively short (6-12 hours),4 notwithstanding that longer values (up to 5.4 days) are controversially discussed.5-8 Although neutrophils have originally been considered to be a homogenous population of blood cells, recent studies point to the existence of different phenotypes of these leukocytes.9-14 In this context, neutrophils aged in the circulation seem to be of particular relevance because these immune cells are critically involved in the mobilization of nonaged neutrophils from the bone marrow under steady-state conditions15-17 : in a microbiota-driven process,18 ageing neutrophils upregulate the chemokine receptor CXCR4 on their cell surface, which allows them to home back to the bone marrow (via the chemokine CXCL12), ultimately resulting in the clearance of these leukocytes by resident macrophages.19 In a negative-feedback mechanism, these events regulate granulopoiesis through the interleukin-17 (IL-17)/granulocyte colony-stimulating factor axis.16 Moreover, phagocytosis of aged neutrophils by bone marrow macrophages modulates the size and function of the hematopoietic niche in a liver X receptor–dependent manner, thereby controlling hematopoietic stem and progenitor cell homeostasis.15 The role of aged neutrophils under inflammatory conditions, however, is poorly understood.
In the present study, we uncovered a distinct, highly reactive phenotype of aged neutrophils that enables these immune cells to serve as part of the first line of defense in inflammatory reactions. Ageing in the circulation might therefore be crucial for neutrophils to effectively fulfill their task in host defense.
Methods
A more detailed description of the methods used in this study can be found in supplemental Methods, available on the Blood Web site.
Animals
Male wild-type (WT) C57BL/6NCrl mice and C57BL/6J mice were purchased from Charles River (Sulzfeld, Germany). Male Toll-like receptor-2 (TLR-2)-deficient mice (B6.129-Tlr2tm1Kir/J; backcrossed 9 generations to C57BL/6J), Tlr4-mutated mice (B6.B10ScN-Tlr4lps-del/JthJ; backcrossed 5 generations to C57BL/6J), and P-selectin/CD62P-deficient (SELP−/−) (B6.129s7-Selp/J; backcrossed 10 generations to C57BL/6J) mice were purchased from The Jackson Laboratory via Charles River.
The experiments of the present study were performed with mice 10-15 weeks old (6 weeks old in selected experiments). Animals were housed under conventional conditions, including food (ssniff, Soest, Germany) and water ad libitum. All experiments were performed according to the German Animal Welfare Law and approved by the Government of Upper Bavaria.
Anesthesia
Mice were anesthetized by intraperitoneal (IP) injection of a ketamine/xylazine mixture (100 mg/kg ketamine and 10 mg/kg xylazine).
In vivo BrdU labeling of endogenous neutrophils
Neutrophil precursors in the mouse bone marrow were labeled via a single IV injection of 5-bromo-2′-deoxyuridine (BrdU; 2.5 mg per mouse; FITC (fluorescein isothiocyanate) BrdU Flow Kit; BD Biosciences, San Jose, CA), as described previously.15 This approach allows the differentiation of circulating aged neutrophils from nonaged neutrophils 48 hours after BrdU labeling via flow cytometry.
Experimental groups
Animals were assigned randomly to the following groups: in a first set of experiments, trafficking of aged neutrophils was analyzed in WT, TLR-2-deficient, and TLR-4-mutant mice receiving an IP injection of 500 µg lipopolysaccharide (LPS) or vehicle, and in WT mice receiving an IP injection of 500 µg LPS or vehicle plus an intra-arterial injection of anti-CXCL12 monoclonal antibodies (mAbs), a CXCR4 inhibitor, a TLR-4 inhibitor, or isotype control antibodies/vehicle (n = 4-5). In addition, trafficking of aged neutrophils was analyzed upon IP injection of 25 µg LPS or in WT mice at age 6 weeks upon IP injection of 500 µg LPS.
In further experiments, endothelial cell interactions and transmigration events of aged and nonaged neutrophils were analyzed in the cremaster muscle of WT mice receiving differentially labeled neutrophils isolated from WT (nonaged neutrophils) or P-selectin/CD62P-deficient (SELP−/−) animals (aged neutrophils; n = 4).
Experimental protocols
To study the trafficking of aged neutrophils to bone marrow, blood, spleen, liver, lungs, and kidneys during endotoxemia, anesthetized WT, TLR-2-deficient, or TLR-4-mutant mice received an IP injection of 25 µg or 500 µg LPS or vehicle 3 hours before harvesting bone marrow, blood, and organs. After organ lysis, the number of aged neutrophils was analyzed in the samples by an automated cell counter and multichannel flow cytometry. In selected experiments, WT mice received inhibitors or blocking antibodies via the tail vein 30 minutes before LPS stimulation.
To analyze intravascular rolling, adherence, and transmigration of aged and nonaged neutrophils, neutrophils were isolated from the whole blood of anesthetized donor WT or P-selectin/CD62P-deficient (SELP−/−) mice. Upon differential fluorescence labeling, anesthetized WT recipient mice received an intra-arterial injection of the cells 1 hour before surgical preparation of the cremaster muscle. After baseline in vivo microscopy measurements, the cremaster muscle was superfused with a 100 nM LPS solution. In vivo microscopy recordings were repeated at 30, 60, 90, 120, 150, and 180 minutes after the onset of inflammation.
Endotoxemia
Endotoxemia was induced in mice by an IP injection of 25 µg or 500 µg LPS (from Escherichia coli 0111:B4, purified by phenol extraction; Sigma-Aldrich Chemie GmbH, Taufkirchen, Germany; suspended in 200 µL phosphate-buffered saline).
Cremaster muscle assay
The mouse cremaster muscle was surgically prepared as described by Baez,20 with minor modifications (see supplemental Methods).
In vivo microscopy
The setup for in vivo microscopy was centered around an Axio Scope.A1 microscope equipped with a Colibiri 2 LED light source (Carl Zeiss Microscopy GmbH, Göttingen, Germany) for fluorescence epi-illumination microscopy. To differentiate 2 neutrophil populations stained with either CellTracker Green CMFDA or Red CMTPX (Thermo Fisher Scientific, Waltham, MA), light with the wavelength of 485/20 nm (CellTracker Green) and of 560/25 nm (CellTracker Red) was used in combination with a QUAD filter set (QUAD DAPI (4′,6-diamidino-2-phenylindole)/FITC (fluorescein isothiocyanate)/Cy3/Cy5 sbx HC Filterset; AHF Analysentechnik AG, Tübingen, Germany). Additionally, red light (≥670 nm) was applied to obtain transillumination images of the tissue, as described previously.21
Isolation of blood neutrophils
Whole blood was harvested from anesthetized WT mice via the vena cava. Blood samples were cleared from red blood cells using HetaSep (STEMCELL Technologies SARL, Germany). As described previously,22 the EasySep Mouse Neutrophil Enrichment Kit (STEMCELL Technologies SARL), in combination with 2 EasySep Magnets (STEMCELL Technologies SARL), was used to isolate neutrophils by negative selection, resulting in a neutrophil suspension with a purity of >90%.
Conformation status of integrins
To assess the conformational status of integrins on aged and nonaged mouse neutrophils, anticoagulated peripheral blood of WT mice was suspended in Hanks balanced salt solution containing 1 mM CaCl2 and MgCl2 (Life Technologies, Carlsbad, CA), and binding of ICAM-1/Fc or VCAM-1/Fc (10 μg/mL; R&D Systems) was analyzed by multichannel flow cytometry, as described before.23
The conformation-specific antibodies mAb24 (high-affinity conformation of β2 integrins; mouse anti-human, monoclonal; Abcam, Cambridge, United Kingdom) and KIM127 (intermediate-affinity conformation of β2 integrins; mouse anti-human, monoclonal; courtesy of M. Sperandio) were used to analyze the integrin conformation status in human neutrophils by multichannel flow cytometry, as described previously.24
Phagocytosis
To assess the phagocytic capacity of aged and nonaged neutrophils in vitro, engulfment of pHrodo Green E coli or pHrodo Green Staphylococcus aureus BioParticles (2 mg/mL; Thermo Fisher Scientific) by neutrophils isolated from the peripheral blood of WT mice was analyzed by multichannel flow cytometry, as described previously.25
To analyze the phagocytic capacity of aged and nonaged neutrophils in vivo, 200 µL of pHrodo Green E coli BioParticles Conjugate (2 mg/mL; Thermo Fisher Scientific) was administered via the tail vein of endotoxemic WT mice. After 60 minutes, bioparticle engulfment in neutrophils isolated from blood, bone marrow, and solid organs was analyzed by multichannel flow cytometry.
Statistics
Data analysis was performed with a statistical software package (SigmaStat for Windows; Jandel Scientific) employing the Mann-Whitney rank-sum test (2 groups) or analysis of variance on ranks test, followed by the Dunnett test (>2 groups) for the estimation of stochastic probability in intergroup comparisons. Mean values and standard error of the mean are given. P values <.05 were considered significant.
Results
Trafficking of aged neutrophils in the acute inflammatory response
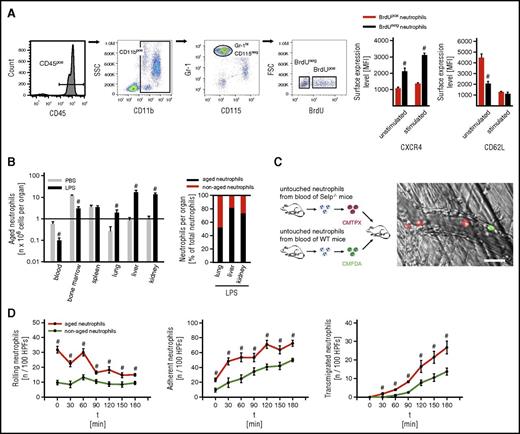
In a first approach, we sought to characterize the fate of aged neutrophils in the acute inflammatory response. For this purpose, leukocyte counts were determined in bone marrow, blood, and lysates of different organs harvested from unstimulated and endotoxemic WT mice and further differentiated by flow cytometry: after identifying myeloid leukocytes (CD45+ and CD11b+ cells), neutrophils were differentiated from monocytes by high expression of Gr-1 and lack of expression of CD115, as demonstrated elsewhere.18,26 This gating strategy was validated by using the neutrophil marker Ly-6G (supplemental Figures 1 and 2). Metabolic cell labeling with fluorescence-labeled BrdU was employed for the determination of the neutrophil ageing status by measuring the nuclear fluorescence signal in these immune cells: 48 hours after systemic application of BrdU, aged neutrophils were easily identified as neutrophils that have not integrated BrdU in their DNA due to their postmitotic state in the labeling period. Nonaged neutrophils, however, appear as BrdU-positive because these cells have incorporated BrdU during mitosis in the labeling period. Thus, aged (BrdUneg CXCR4hi) and nonaged (BrdUpos CXCR4lo) Gr-1hi CD115neg neutrophils were identified by pulse labeling with the thymidine analog BrdU, as well as by the expression of the chemokine receptor CXCR4, as demonstrated previously.15,18
Consistent with previous reports,15,18 surface levels of L-selectin/CD62L were found to be low on BrdUneg Gr-1hi CD115neg neutrophils and high on BrdUpos Gr-1hi CD115neg neutrophils in the steady state. Under inflammatory conditions, however, surface expression of L-selectin/CD62L did not differ between BrdUpos and BrdUneg Gr-1hi CD115neg neutrophils and was therefore not used as a marker for the ageing status of neutrophils (Figure 1A).
Trafficking of aged neutrophils during inflammation. Employing multichannel flow cytometry, aged and nonaged neutrophils isolated from WT mice were differentiated by pulse labeling with BrdU as detailed in “Methods.” (A) Gating strategy and results for the expression of CXCR4 and L-selectin/CD62L on aged (BrdUneg) and nonaged (BrdUpos) neutrophils from the peripheral blood of WT mice upon exposure to PBS or LPS (mean ± SEM for n = 4; #P < .05 vs nonaged neutrophils). (B) Results for numbers of aged and nonaged neutrophils in bone marrow, blood, and lysates of different organs of WT mice upon IP injection of PBS or LPS (mean ± SEM for n = 5 ; #P < .05 vs PBS). (C) Using multichannel in vivo microscopy on the cremaster muscle of WT recipient mice, recruitment dynamics of adoptively transferred fluorescence-labeled neutrophils isolated from WT or P-selectin/CD62P-deficient (SELP−/−) donor mice were analyzed upon stimulation with LPS. A representative in vivo microscopy image shows aged neutrophils in red and nonaged neutrophils in green (bar represents 20 µm). (D) Results for numbers of intravascularly rolling, adherent, and transmigrated aged or nonaged neutrophils (mean ± SEM for n = 4; #P < .05 vs nonaged neutrophils). FSC, forward scatter; HPFs, high-power fields; MFI, mean fluorescence intensity; PBS, phosphate-buffered saline; SEM, standard error of the mean; SSC, side scatter.
Trafficking of aged neutrophils during inflammation. Employing multichannel flow cytometry, aged and nonaged neutrophils isolated from WT mice were differentiated by pulse labeling with BrdU as detailed in “Methods.” (A) Gating strategy and results for the expression of CXCR4 and L-selectin/CD62L on aged (BrdUneg) and nonaged (BrdUpos) neutrophils from the peripheral blood of WT mice upon exposure to PBS or LPS (mean ± SEM for n = 4; #P < .05 vs nonaged neutrophils). (B) Results for numbers of aged and nonaged neutrophils in bone marrow, blood, and lysates of different organs of WT mice upon IP injection of PBS or LPS (mean ± SEM for n = 5 ; #P < .05 vs PBS). (C) Using multichannel in vivo microscopy on the cremaster muscle of WT recipient mice, recruitment dynamics of adoptively transferred fluorescence-labeled neutrophils isolated from WT or P-selectin/CD62P-deficient (SELP−/−) donor mice were analyzed upon stimulation with LPS. A representative in vivo microscopy image shows aged neutrophils in red and nonaged neutrophils in green (bar represents 20 µm). (D) Results for numbers of intravascularly rolling, adherent, and transmigrated aged or nonaged neutrophils (mean ± SEM for n = 4; #P < .05 vs nonaged neutrophils). FSC, forward scatter; HPFs, high-power fields; MFI, mean fluorescence intensity; PBS, phosphate-buffered saline; SEM, standard error of the mean; SSC, side scatter.
According to previously published studies,27,28 leukocyte trafficking was analyzed 3 hours after IP injection of a high dose of LPS, because lower doses of LPS elicited rather weak leukocyte responses in this acute phase of the inflammatory reaction (supplemental Figure 3). Based on these protocols, mice older than 10 weeks were used in our experiments and exhibited comparable responses of aged neutrophils during endotoxemia as younger mice (supplemental Figure 2).
Upon induction of endotoxemia, significantly fewer aged neutrophils were found in bone marrow and peripheral blood as compared with unstimulated mice. In contrast, significantly more aged neutrophils were detected in liver, lungs, and kidneys of endotoxemic mice than in unstimulated controls (Figure 1B).
Using multichannel in vivo microscopy on the cremaster muscle of WT mice, the dynamics of the initial endothelial cell interactions of aged and nonaged neutrophils were evaluated in further detail. In adoptive cell transfer experiments, fluorescence-labeled total neutrophils isolated from P-selectin-deficient donor mice (exhibiting >85% BrdUneg CXCR4hi [aged] neutrophils due to impaired recruitment of these aged immune cells back to the bone marrow18 ) were found to roll and firmly adhere in postcapillary venules, as well as to transmigrate into the perivascular tissue in the LPS-stimulated cremaster muscle of WT recipient mice as the first leukocytes. Later on, delayed responses of fluorescence-labeled total neutrophils isolated from WT donor mice (exhibiting ∼20% BrdUneg CXCR4high [aged] neutrophils) were observed (Figure 1C-D; supplemental Videos 1 and 2).
Surface expression of adhesion and signaling molecules on aged neutrophils
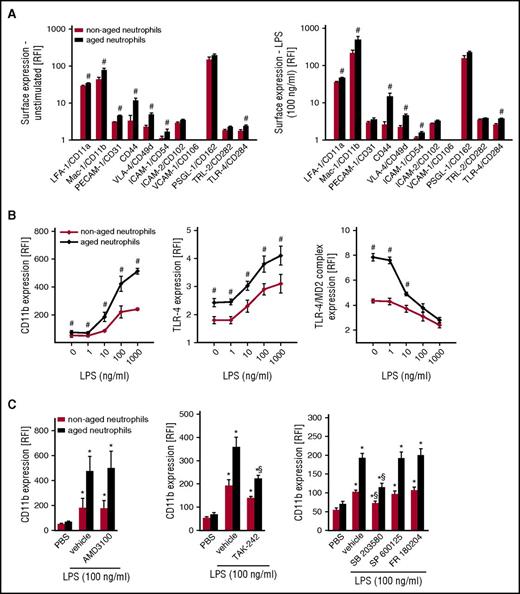
Neutrophil recruitment to the site of inflammation is mediated by specific adhesion and signaling molecules.29-34 To better understand the mechanisms underlying the trafficking of aged neutrophils to inflamed tissues, we first sought to characterize the consequences of ageing on the surface expression of key adhesion and signaling molecules involved in the leukocyte extravasation process. Employing multichannel flow cytometry, common and distinct expression patterns of different adhesion and signaling molecules on the surface of aged and nonaged neutrophils were detected. Specifically, surface expression levels of ICAM-2/CD102, VCAM-1/CD106, P-selectin glycoprotein ligand-1(PSGL-1)/CD162, or TLR-2/CD282 did not significantly differ between aged and nonaged neutrophils isolated from the peripheral blood of unstimulated WT mice. In contrast, surface expression levels of lymphocyte function–associated antigen-1 (LFA-1)/CD11a, macrophage-1 antigen (Mac-1)/CD11b, PECAM-1/CD31, CD44, very-late-activation antigen-4 (VLA-4)/CD49d, ICAM-1/CD54, and TLR-4/CD284 were significantly higher on the surface of aged neutrophils than on nonaged neutrophils (Figure 2A). Of interest, surface expression of Mac-1/CD11b and TLR-4 further increased on aged neutrophils when stimulated with LPS (Figure 2A-B), lipoteichoic acid (LTA), or high-mobility group protein 1 (HMGB1; supplemental Figure 4) as compared with unstimulated conditions, whereas surface expression of the TLR-4/MD-2 complex decreased. In contrast, surface expression levels of LFA-1/CD11a, PECAM-1/CD31, CD44, VLA-4/CD49d, ICAM-2/CD102, VCAM-1/CD106, PSGL-1/CD162, and TLR-2/CD282 remained largely unaltered (Figure 2A-B). This upregulation of Mac-1/CD11b on aged neutrophils required TLR-4- and p38 MAPK-dependent (but not JNK- or extracellular signal-regulated kinase 1/2 [ERK1/2] MAPK- and CXCR4-dependent) signaling events (Figure 2C).
Surface expression of adhesion and signaling molecules on aged neutrophils. Using multichannel flow cytometry, surface expression of different adhesion and signaling molecules on aged neutrophils was analyzed as detailed in “Methods.” Quantitative data for aged and nonaged neutrophils isolated from the peripheral blood of WT mice undergoing exposure to PBS or LPS (A, B), as well as to the CXCR4 inhibitor AMD3100, the TLR-4 inhibitor TAK-242, or the MAPK inhibitor compounds FR180204 (ERK 1/2 MAPK), SB203580 (p38 MAPK), or SP600125 (JNK MAPK) (C). Mean ± SEM for n = 4-6; #P < .05 vs nonaged neutrophils; *P < .05 vs PBS; §P < .05 vs drug vehicle. RFI, relative fluorescence intensity.
Surface expression of adhesion and signaling molecules on aged neutrophils. Using multichannel flow cytometry, surface expression of different adhesion and signaling molecules on aged neutrophils was analyzed as detailed in “Methods.” Quantitative data for aged and nonaged neutrophils isolated from the peripheral blood of WT mice undergoing exposure to PBS or LPS (A, B), as well as to the CXCR4 inhibitor AMD3100, the TLR-4 inhibitor TAK-242, or the MAPK inhibitor compounds FR180204 (ERK 1/2 MAPK), SB203580 (p38 MAPK), or SP600125 (JNK MAPK) (C). Mean ± SEM for n = 4-6; #P < .05 vs nonaged neutrophils; *P < .05 vs PBS; §P < .05 vs drug vehicle. RFI, relative fluorescence intensity.
Integrin affinity changes in aged neutrophils
Intravascular adherence and the subsequent transmigration of neutrophils to the perivascular tissue require the interplay of β2 integrins such as Mac-1/CD11b, with its endothelial binding partner of the immunoglobulin superfamily ICAM-1/CD54. To enable these interactions, surface-expressed integrins have to be activated to higher-affinity conformations.29-34 With respect to the immediate recruitment of aged neutrophils upon onset of inflammation, we hypothesized that this subset of immune cells exceptionally effectively transduces inflammatory signals into conformational changes of β2 integrins. As a measure of affinity changes of β2 integrins, the binding capacity for their interaction partner ICAM-1/CD54 was determined. Under unstimulated conditions and upon stimulation with LPS (Figure 3A), LTA, or HMGB1 (supplemental Figure 5), binding of ICAM-1/CD54 to the surface of aged neutrophils was significantly higher than on nonaged neutrophils. These inflammatory changes were dependent on TLR-4 and on p38 MAPK, but not on JNK or ERK1/2 MAPK. In experiments with human neutrophils, stimulation with LPS induced a significant increase in the binding of the antibody mAb 24 (indicating the high-affinity conformation of β2 integrins), but not of the antibody kim127 (indicative for the intermediate affinity of β2 integrins), as compared with unstimulated conditions. This increase in the binding of mAb 24 was significantly higher in aged neutrophils than in nonaged neutrophils (Figure 3B).
Integrin affinity changes in aged neutrophils. Using multichannel flow cytometry, binding of the β2 integrin ligand ICAM-1/CD54 to aged and nonaged neutrophils isolated from the peripheral blood of WT mice was analyzed as detailed in “Methods.” (A) Representative flow cytometry histogram and quantitative data for neutrophils isolated from the peripheral blood of WT mice undergoing exposure to PBS or LPS, as well as to the TLR-4 inhibitor TAK-242 or the MAPK inhibitor compounds FR180204 (ERK 1/2 MAPK), SB203580 (p38 MAPK), or SP600125 (JNK 1/2/3 MAPK). Mean ± SEM for n = 4-6; *P < .05 vs PBS; §P < .05 vs drug vehicle. (B) Representative flow cytometry histogram and quantitative results for binding of the antibodies kim127 (indicating intermediate-affinity conformation of β2 integrins) and mAb 24 (indicating high-affinity conformation of β2 integrins) to human aged and nonaged neutrophils isolated from the peripheral blood of healthy volunteers and stimulated with PBS or LPS. Mean ± SEM for n = 4-6; #P < .05 vs nonaged. AF, Alexa Fluor; PE, phycoerythrin.
Integrin affinity changes in aged neutrophils. Using multichannel flow cytometry, binding of the β2 integrin ligand ICAM-1/CD54 to aged and nonaged neutrophils isolated from the peripheral blood of WT mice was analyzed as detailed in “Methods.” (A) Representative flow cytometry histogram and quantitative data for neutrophils isolated from the peripheral blood of WT mice undergoing exposure to PBS or LPS, as well as to the TLR-4 inhibitor TAK-242 or the MAPK inhibitor compounds FR180204 (ERK 1/2 MAPK), SB203580 (p38 MAPK), or SP600125 (JNK 1/2/3 MAPK). Mean ± SEM for n = 4-6; *P < .05 vs PBS; §P < .05 vs drug vehicle. (B) Representative flow cytometry histogram and quantitative results for binding of the antibodies kim127 (indicating intermediate-affinity conformation of β2 integrins) and mAb 24 (indicating high-affinity conformation of β2 integrins) to human aged and nonaged neutrophils isolated from the peripheral blood of healthy volunteers and stimulated with PBS or LPS. Mean ± SEM for n = 4-6; #P < .05 vs nonaged. AF, Alexa Fluor; PE, phycoerythrin.
Role of TLR-4 and Mac-1/CD11b on trafficking of aged neutrophils to the site of inflammation
Because activation of aged neutrophils induced the expression of Mac-1/CD11b on their cell surface in a TLR-4-dependent manner, we hypothesized that this particular pathway is functionally relevant for the trafficking of aged neutrophils to the site of inflammation. As demonstrated above, endotoxemia induced a significant elevation in numbers of aged neutrophils accumulated in liver, lungs, and kidneys as compared with unstimulated control animals, whereas numbers of aged neutrophils in bone marrow and blood were significantly diminished. These inflammatory changes were almost completely abolished in WT mice receiving blocking mAbs directed against Mac-1/CD11b or in TLR-4-mutant mice, and were significantly reduced in TLR-2-deficient mice or in WT mice treated with a TLR-4 inhibitor (TAK-242). Remarkably, inhibition of the chemokine receptor CXCR4 (by compound AMD3100) or antibody-mediated blockade of its canonical ligand stromal cell-derived factor 1-α (SDF1-α)/CXCL12 did not affect the trafficking of aged neutrophils to peripheral tissues in endotoxemia (Figure 4; supplemental Figure 6).
Mechanisms underlying trafficking of aged neutrophils during inflammation. Using multichannel flow cytometry, numbers of aged and nonaged neutrophils in bone marrow, blood, and lysates of different organs of WT, TLR-2−/−, or TLR-4-mutant mice and of WT mice receiving intra-arterial injections of the CXCR4 inhibitor AMD3100, blocking anti-CXCL12 mAbs, the TLR-4 inhibitor TAK-242, or drug vehicle/isotype control antibodies upon IP injection of PBS or LPS were quantitatively analyzed as detailed in “Methods.” Results that have been normalized to controls are shown. Mean ± SEM for n = 4-5; #P < .05 vs PBS; *P < .05 vs drug vehicle/isotype control/WT.
Mechanisms underlying trafficking of aged neutrophils during inflammation. Using multichannel flow cytometry, numbers of aged and nonaged neutrophils in bone marrow, blood, and lysates of different organs of WT, TLR-2−/−, or TLR-4-mutant mice and of WT mice receiving intra-arterial injections of the CXCR4 inhibitor AMD3100, blocking anti-CXCL12 mAbs, the TLR-4 inhibitor TAK-242, or drug vehicle/isotype control antibodies upon IP injection of PBS or LPS were quantitatively analyzed as detailed in “Methods.” Results that have been normalized to controls are shown. Mean ± SEM for n = 4-5; #P < .05 vs PBS; *P < .05 vs drug vehicle/isotype control/WT.
Production of ROS and cytokine synthesis in aged neutrophils
Having arrived at the site of inflammation, leukocytes employ their individual functional properties for their fight against invading pathogens. The intracellular production of reactive oxygen species (ROS) is an integral part of the host defense apparatus of phagocytes and plays a pivotal role in cell signaling.35 To measure intracellular ROS production, multichannel flow cytometry analyses of neutrophils isolated from the peripheral blood of WT mice were performed. Under unstimulated conditions or upon stimulation with LPS, mitochondrial/nuclear and cytosolic ROS production did not differ between aged and nonaged neutrophils (Figure 5A).
Intracellular ROS and cytokine production in aged neutrophils. (A) Representative microscopy images illustrating mitochondrial/nuclear and cytosolic ROS generation in unstimulated and LPS-stimulated neutrophils (bars represent 5 µm). Using multichannel flow cytometry, intracellular production of ROS (A) and cytokines (B) was quantitatively analyzed in aged and nonaged neutrophils isolated from the peripheral blood of WT mice as detailed in “Methods.” Quantitative data for neutrophils undergoing exposure to PBS (unstimulated) or LPS are shown. Mean ± SEM for n = 4-6. IFN, interferon; TNF, tumor necrosis factor.
Intracellular ROS and cytokine production in aged neutrophils. (A) Representative microscopy images illustrating mitochondrial/nuclear and cytosolic ROS generation in unstimulated and LPS-stimulated neutrophils (bars represent 5 µm). Using multichannel flow cytometry, intracellular production of ROS (A) and cytokines (B) was quantitatively analyzed in aged and nonaged neutrophils isolated from the peripheral blood of WT mice as detailed in “Methods.” Quantitative data for neutrophils undergoing exposure to PBS (unstimulated) or LPS are shown. Mean ± SEM for n = 4-6. IFN, interferon; TNF, tumor necrosis factor.
Similarly, synthesis of proinflammatory (IL-1α, IL-1β, IL-6, tumor necrosis factor, and interferon-γ) and anti-inflammatory (IL-4, IL-10, and interferon-α) cytokines was significantly increased in aged or nonaged neutrophils upon stimulation with LPS, but did not significantly vary between both populations (Figure 5B).
Phagocytic behavior of aged neutrophils
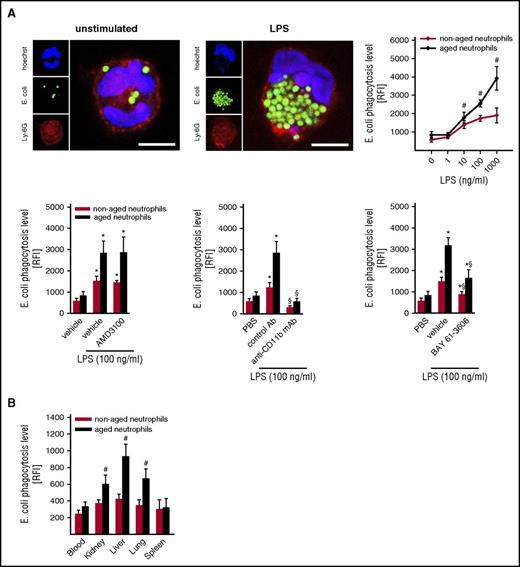
To evaluate the phagocytic behavior of aged and nonaged neutrophils, phagocytosis of E coli and S aureus bioparticles was analyzed in vitro by flow cytometry in neutrophils isolated from WT mice. Under unstimulated conditions, phagocytosis of bioparticles did not significantly differ between aged and nonaged neutrophils. Upon cell activation, however, aged neutrophils exhibited a significantly higher phagocytic potential as compared with nonaged neutrophils. This particular phagocytic behavior of aged neutrophils was dependent on the phagocytosis receptor Mac-1/CD11b and spleen tyrosine kinase–dependent signaling events (Figure 6A; supplemental Figure 7).
Phagocytic potential of aged neutrophils. The phagocytic potential of aged and nonaged neutrophils for E coli bioparticles was analyzed as detailed in “Methods.” (A) Representative confocal microscopy images illustrating phagocytosis of E coli bioparticles by unstimulated and LPS-stimulated neutrophils (bars represent 5 µm). Quantitative data for aged and nonaged neutrophils isolated from the peripheral blood of unstimulated WT mice upon exposure to PBS or varying concentrations of LPS, as well as to the CXCR4 inhibitor AMD3100, the TLR-4 inhibitor TAK-242, blocking anti-Mac-1/CD11b mAbs, the spleen tyrosine kinase inhibitor BAY 61-3606, or drug vehicle/isotype control (mean ± SEM for n = 4; #P < .05 vs nonaged; *P < .05 vs PBS; §P < .05 vs drug vehicle/isotype control). (B) Quantitative data for aged and nonaged neutrophils isolated from the blood or from lysates of different organs of WT mice undergoing intra-arterial injection of E coli bioparticles and IP injection of LPS (mean ± SEM for n = 5; #P < .05 vs nonaged neutrophils).
Phagocytic potential of aged neutrophils. The phagocytic potential of aged and nonaged neutrophils for E coli bioparticles was analyzed as detailed in “Methods.” (A) Representative confocal microscopy images illustrating phagocytosis of E coli bioparticles by unstimulated and LPS-stimulated neutrophils (bars represent 5 µm). Quantitative data for aged and nonaged neutrophils isolated from the peripheral blood of unstimulated WT mice upon exposure to PBS or varying concentrations of LPS, as well as to the CXCR4 inhibitor AMD3100, the TLR-4 inhibitor TAK-242, blocking anti-Mac-1/CD11b mAbs, the spleen tyrosine kinase inhibitor BAY 61-3606, or drug vehicle/isotype control (mean ± SEM for n = 4; #P < .05 vs nonaged; *P < .05 vs PBS; §P < .05 vs drug vehicle/isotype control). (B) Quantitative data for aged and nonaged neutrophils isolated from the blood or from lysates of different organs of WT mice undergoing intra-arterial injection of E coli bioparticles and IP injection of LPS (mean ± SEM for n = 5; #P < .05 vs nonaged neutrophils).
In line with our in vitro results, phagocytosis of E coli bioparticles by aged neutrophils in the inflamed liver, lungs, and kidneys of endotoxemic WT mice was significantly higher than by nonaged neutrophils. Importantly, phagocytosis of E coli bioparticles in peripheral blood and spleen was low and did not differ between aged and nonaged neutrophils (Figure 6B).
Discussion
Due to their ability to migrate to the site of inflammation and to eliminate invading pathogens, neutrophils play a substantial role in the immune response.1,2 Although these leukocytes have been proposed to represent a rather homogeneous population of immune cells, there is emerging evidence of the existence of different neutrophil phenotypes.9-14 In the peripheral blood, heterogeneity of neutrophils arises from ageing of circulating neutrophils and the concomitant release of nonaged neutrophils from the bone marrow: in a microbiota-driven process, ageing neutrophils gradually upregulate the chemokine receptor CXCR4 on their cell surface during intravascular margination in peripheral organs, which allows the clearance of these leukocytes in bone marrow, liver, and spleen via the chemokine CXCL12/SDF-1α.17,36-39 These events promote an IL-17/granulocyte colony-stimulating factor–mediated feedback inhibition of neutrophil production in the bone marrow and cause the rhythmic modulation of the hematopoietic stem cell niche in the steady state.15,16 The fate of aged neutrophils under inflammatory conditions, however, remains largely unclear.
In a first approach, we therefore sought to follow the routes aged neutrophils take in the acute inflammatory response. In our fate-mapping experiments, significantly fewer aged neutrophils were detected in the bone marrow of endotoxemic mice than in unstimulated controls. Conversely, the numbers of aged neutrophils in liver, lungs, and kidneys dramatically increased upon induction of endotoxemia, collectively indicating that in the acute inflammatory response, aged neutrophils cease returning to the bone marrow and start trafficking to the site of inflammation. To evaluate the recruitment of aged neutrophils to inflamed peripheral tissues in further detail, we performed multichannel in vivo microscopy analyses. In these adoptive cell transfer experiments, aged neutrophils were observed to instantly accumulate in inflamed microvessels and the surrounding perivascular space, right before nonaged neutrophils. Thus, aged neutrophils completely change their migratory behavior in the organism under inflammatory conditions, allowing them to reach inflamed tissues among the first immune cells.
Toward a more comprehensive, mechanistic understanding of these events, we sought to characterize phenotypic and functional properties of aged and nonaged neutrophils. In a first step, the expression patterns of major adhesion and signaling molecules involved in leukocyte trafficking29-34 were analyzed. We found that ageing in the circulation does not significantly alter the expression levels of PSGL-1/CD162 (a major selectin ligand), ICAM-2/CD102 and VCAM-1/CD106 (members of the immunoglobulin superfamily), or the damage- and pathogen-associated molecular pattern receptor TLR-2 on neutrophils. In contrast, expression of the integrins LFA-1/CD11a, Mac-1/CD11b, and VLA-4/CD49d; the glycoprotein CD44; the immunoglobulin superfamily members PECAM-1/CD31 and ICAM-1/CD54; as well as TLR-4 was significantly higher on aged neutrophils than on nonaged neutrophils, extending previously published observations.15,18 Most important, however, surface expression levels of the β2 integrin Mac-1/CD11b in aged neutrophils rose even further when challenged with inflammatory mediators, whereas nonaged neutrophils showed comparatively weak responses. These data point to a particularly high responsiveness of aged neutrophils toward various inflammatory stimuli, including LPS (a component of various gram-negative bacteria40 ), LTA (a component of various gram-positive bacteria41 ), and HMGB1 (a potent inflammatory mediator released in sterile injury42 ), that is mediated through TLR-4- and p38 MAPK-dependent signaling events. Noteworthy is that activation of aged neutrophils did not require JNK/ERK1/2 MAPK or the chemokine receptor CXCR4 that serves as an alternative receptor of LPS43 and coordinates the SDF-1α/CXCL12-dependent recruitment of aged neutrophils back to bone marrow, liver, and spleen in the steady state.36,37,39
Integrins are transmembrane proteins that facilitate cell-cell and cell-extracellular matrix interactions. Adhesion of neutrophils to the microvascular endothelium and the subsequent transmigration of these immune cells to the perivascular tissue require the activation of leukocyte β2 integrins (such as Mac-1/CD11b) to higher-affinity conformations, which enables interactions with endothelially expressed ICAM-1/CD54.29-34 Here, we demonstrate that β2 integrins on aged and nonaged neutrophils exhibit a similar activation status under unstimulated conditions. Upon cell activation, however, aged neutrophils expressed significantly higher levels of β2 integrins in high-affinity conformation as compared with nonaged neutrophils, a process that required TLR-4- and p38 MAPK-dependent signaling events. Hence, aged neutrophils show a specific, highly reactive phenotype that enables these aged leukocytes to promptly respond to inflammatory stimuli, rather than just representing an excessive activation status of these immune cells.
With respect to these findings and the well-known roles of TLR-4 and CD11b/Mac-1 in leukocyte trafficking,29-34 we hypothesized that these molecules are particularly relevant for the migration of aged neutrophils to the site of inflammation. In a further series of experiments, the recruitment of aged neutrophils to liver, lungs, and kidneys in endotoxemia was found to be almost completely abolished in TLR-4-mutant mice and in WT mice treated with blocking antibodies directed against CD11b/Mac-1, and was significantly diminished in TLR-2-deficient mice. In this context, it must be considered that TLR-2-deficient and TLR-4-mutant mice exhibit only very low numbers of circulating aged neutrophils as compared with WT animals (due to the involvement of these receptors in microbiota-dependent ageing18 ), complicating the interpretation of these results. Because application of a TLR-4 inhibitor in WT mice right before the onset of inflammation significantly reduced the migration of aged neutrophils to peripheral organs, however, we collectively conclude that the trafficking of these aged immune cells under inflammatory conditions critically involves TLR-4 and Mac-1/CD11b. Notably, inhibition of the chemokine receptor CXCR4 or its ligand SDF-1α/CXCL12, which regulates the return of aged neutrophils to clearance organs in the steady state,15,18,36,37,39 did not significantly alter the recruitment of aged neutrophils to inflamed tissue. In summary, our data indicate that neutrophils aged in the circulation instantly recognize and effectively translate inflammatory signals into conformational changes of the β2 integrin Mac-1/CD11b through TLR-4 and p38 MAPK, enabling these “experienced” immune cells to rapidly navigate to the site of inflammation (Figure 7).
Schematic illustration of the fate of aged neutrophils in homeostasis and inflammation. In the steady state, nonaged neutrophils are released from the bone marrow into the circulation before these immune cells age during intravascular margination in peripheral organs. Ultimately, aged neutrophils are cleared in bone marrow, liver, and spleen (via SDF-1α/CXCL12 and CXCR4). Upon onset of inflammation, however, aged neutrophils cease returning to the bone marrow and immediately infiltrate inflamed tissues in a TLR-4- and Mac-1/CD11b-dependent manner, thereby being in the first line of defense in the acute inflammatory response.
Schematic illustration of the fate of aged neutrophils in homeostasis and inflammation. In the steady state, nonaged neutrophils are released from the bone marrow into the circulation before these immune cells age during intravascular margination in peripheral organs. Ultimately, aged neutrophils are cleared in bone marrow, liver, and spleen (via SDF-1α/CXCL12 and CXCR4). Upon onset of inflammation, however, aged neutrophils cease returning to the bone marrow and immediately infiltrate inflamed tissues in a TLR-4- and Mac-1/CD11b-dependent manner, thereby being in the first line of defense in the acute inflammatory response.
Having arrived in inflamed tissues, neutrophils immediately engage their effector functions to eliminate invaded pathogens and to enforce the inflammatory reaction.1,2 In our experiments, we did not detect any significant differences between aged and nonaged neutrophils regarding their potential to produce intracellular ROS (which dispose engulfed pathogens and facilitate intra-/intercellular signaling events) or proinflammatory and anti-inflammatory cytokines, emphasizing the functional integrity of these aged immune cells. Of interest, aged neutrophils have recently been reported to exhibit a higher potential to release neutrophil extracellular traps (an extracellular meshwork of DNA fibers that binds pathogens44 ) as compared with nonaged neutrophils.18 Furthermore, mice have been found to be more at risk for sepsis when challenged in their resting phase,45 which coincides with very low numbers of aged neutrophils in the circulation.15 In line with these observations, we demonstrate that aged neutrophils recruited to the site of inflammation exhibit a significantly higher phagocytic activity for bacteria as compared with nonaged neutrophils. In this context, we found that phagocytosis of bacteria by activated aged neutrophils is strictly dependent on the β2 integrin Mac-1/CD11b (also known as phagocytosis receptor35 ) and involved spleen tyrosine kinase–dependent signaling events. Together, our results suggest that ageing in the circulation also promotes specific functional alterations in neutrophils that sharpen the “senses” of these immune cells for their fight against pathogens.
In conclusion, our experimental data uncover a specific, highly reactive phenotype of aged neutrophils that allows these leukocytes to reach the site of inflammation as part of the first defense line, effectively clearing invaded pathogens. These findings indicate that the maturation process of neutrophils is not restricted to a “production phase” in the bone marrow, but also includes a critical “education phase” in the circulation, enabling these immune cells to completely unfold their functions in the front line of the immune response.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported by the Deutsche Forschungsgemeinschaft Sonderforschungsbereich 914 Projects B2 (S.M.) and B3 (C.A.R. and F.K.); Ludwig-Maximilians-Universität München LMUexcellent (C.A.R.); and Friedrich-Baur-Stiftung (B.U.).
Data presented in this study are part of the doctoral thesis of Y.V.
Authorship
Contribution: B.U. and C.A.R. conceived and designed the experiments; B.U., Y.V., G.Z., K.N., K.S., and F.G. performed the experiments; B.U., G.Z., and C.A.R. analyzed the data; and B.U., S.M., F.K., and C.A.R. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Christoph A. Reichel, Walter Brendel Centre of Experimental Medicine and Department of Otorhinolaryngology, Head and Neck Surgery, Klinikum der Universität München, Ludwig-Maximilians-Universität München, Marchioninistr 15, D-81377 Munich, Germany; e-mail: christoph.reichel@med.uni-muenchen.de.