Abstract
The reconstitution of specific immune niches after bone marrow transplant is mediated by donor immune cells repopulating specific microenvironments. Here, we demonstrate that type 2 innate lymphoid cells (ILC2) in the GI tract are rapidly eliminated by irradiation and/or chemotherapy and are not repopulated in the first month post-transplant by donor bone marrow cells. In contrast, ILC2 cells in the lung are not sensitive to conditioning therapy and quickly recover quantitatively post-transplant. Donor ILC2 cell infusion with donor T cells and TCD bone marrow has been shown by us to markedly diminish lethality and clinical score associated with acute GvHD. However, the mechanism for that finding was unclear. Here, we demonstrate that donor ILC2 infusion enhances the persistence of GI tract, but not splenic or pulmonary MDSCs (Fig 1a). This persistence required the expression of IL-13 by the ILC2 cells (Fig 1b). Absence of IL-13 production by ILC2 cells greatly diminished the activity of those cells to prevent GvHD (Fig 1c). ILC2 cell infusion decreased the number of T cells generating IFN-γ and IL-17A in the GI tract. However, we found no difference in the number of IFN-γ or IL-17A generating T cells in the GI tract after the infusion of IL-13-/- ILC2 cells.
To evaluate the function of ILC2 cells in MDSC biology we performed in vitro quantitation of MDSCs in co-culture with ILC2 cells. MDSCs cultured with WT ILC2s, survived significantly better than MDSCs cultured alone, MDSCs cultured with IL-13 and MDSC cultured in the presence of IL-7 and IL-33. In addition, MDSCs co-cultured with WT ILC2s survived significantly better than those cultured with IL-13-/- ILC2s. Surprisingly, enhancement of MDSC survival requires cell-to-cell contact with ILC2s, as seen when either WT or IL-13-/- ILC2s and MDSCs are cultured in plates across semi-permeable membranes.
ILC2s have been shown to have a role in intestinal barrier repair via amphiregulin (Areg). When compared to untreated recipients, ILC2 treated recipients showed significantly enhance barrier function which can be observed as early as 12 days post-transplant and as late as day 20. Interestingly, recipients of Areg deficient ILC2s show improved survival compared to control treatment but diminished survival compared to recipients of WT ILC2 cells.
Several cell types have been shown to suppress aGvHD, including MDSCs and regulatory T cells (Treg). To evaluate the function of ILC2 cells as treatment, we compared them to MDSC and Treg infusion. When used to treat aGvHD on day 7 post-transplant, IL-13 derived MDSCs have no effect on recipient survival. In contrast ILC2 infusion results in a significant prolongation of survival compared to MDSC infusion with approximately 35% of recipient mice surviving past day 50. When compared to Treg infusion (1:1 ratio with Tcons) both ILC2 and Treg infusions block aGvHD. However, unlike ILC2 infusion in which no mice develop P815 tumors post-transplant, 80% of mice given Tregs developed tumors. At a lower Treg dose (1:4) the GvL response was restored but without the beneficial effect on GvHD.
In summary, ILC2s represent a novel cell population that are required for recovery of GI tract homeostasis following allo-SCT. ILC2s play a pivotal role in the development of donor MDSCs and intestinal barrier repair. Additional investigation should be focused on the recovery of specific immunosuppressive cell populations from donor bone marrow cells after allo-SCT.
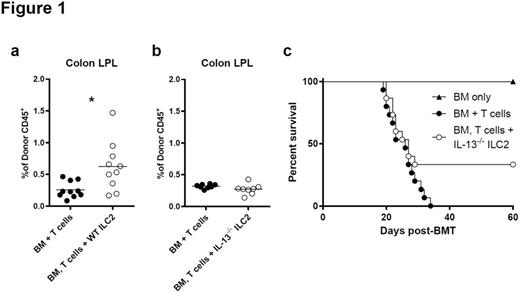
IL-13 dependent ILC2 induced expansion of donor MDSCs. (a) Frequencies CD11b+/GR-1+/Ly-6C+/Ly-6G+ MDSCs in the LP of the colon of allo-SCT recipients 12 days post-transplant with or without WT ILC2s. These represent 2 independent experiments, bar graphs are average ± SEM, student's t test using GraphPad Prism, * p<0.05. (b) Frequency of MDSCs in the LP of the colon of allo-SCT recipients with or without IL-13-/- ILC2s. These represent 2 independent experiments, bar graphs are average ± SEM. (c) B6D2 recipients of B6 T cell depleted bone marrow (BM only), BM plus splenic T cells (BM + T cells) or BM plus T cells with cultured IL-13-/- ILC2 (BM, T cell + IL-13-/- ILC2) were evaluated for percent survival following allo-SCT, one representative of 2 combined experiments shown (n=8 per group in each experiment) p=0.1, by Log-rank (Mantel-cox) test.
IL-13 dependent ILC2 induced expansion of donor MDSCs. (a) Frequencies CD11b+/GR-1+/Ly-6C+/Ly-6G+ MDSCs in the LP of the colon of allo-SCT recipients 12 days post-transplant with or without WT ILC2s. These represent 2 independent experiments, bar graphs are average ± SEM, student's t test using GraphPad Prism, * p<0.05. (b) Frequency of MDSCs in the LP of the colon of allo-SCT recipients with or without IL-13-/- ILC2s. These represent 2 independent experiments, bar graphs are average ± SEM. (c) B6D2 recipients of B6 T cell depleted bone marrow (BM only), BM plus splenic T cells (BM + T cells) or BM plus T cells with cultured IL-13-/- ILC2 (BM, T cell + IL-13-/- ILC2) were evaluated for percent survival following allo-SCT, one representative of 2 combined experiments shown (n=8 per group in each experiment) p=0.1, by Log-rank (Mantel-cox) test.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal