Abstract
Background: Indolent lymphomas are characterized by a chronic relapsing-remitting course. Bendamustine-Rituximab (BR) has been shown to improve overall response rate and progression free survival (PFS) in the upfront treatment of patients with indolent B-cell non-Hodgkin lymphoma (iNHL), as compared with conventional chemoimmunotherapy (Rummel et al., 2013; Flinn et al., 2014). The pan-Canadian Oncology Drug Review has recommended publicly funding BR, but concluded there is substantial uncertainty regarding the regimen's cost-effectiveness. The objective of our study was to assess the cost-effectiveness of BR as compared with Rituximab-Cyclophosphamide, Doxorubicin, Vincristine, Prednisone (RCHOP) as frontline treatment for patients with advanced iNHL from a Canadian perspective.
Methods: A Markov model was developed to estimate the costs, life expectancy and quality-adjusted life-years (QALYs) associated with the two regimen options allowing determination of the incremental cost-utility ratio (ICUR). Model parameters were derived from peer-reviewed studies. Key health states included FT (frontline therapy), MR (2-year state of maintenance R), PF1 (1st progression-free state), PD1/2/3 (subsequent progressive disease states requiring salvage), PF2/3/4 (subsequent progression-free states post-salvage), palliation and death. To determine progression after FT, individual data elements were derived from the published literature, and transition probabilities were determined through parametric survival analysis. Age-related mortality was obtained from Statistics Canada. Cost data (in 2016 Canadian dollars) were obtained from current funding arrangements under the New Drug Funding Program of Cancer Care Ontario, the Ontario Health Insurance Plan Schedule of Benefits and Fees, and the published literature. Utility values for health states and utility decrements associated with treatment related adverse events (AEs) were derived from peer-reviewed studies. The analysis was performed from the health care provider perspective, with a lifetime time horizon (equivalent to 24 years) and cycle lengths of 6 months. Patients were treated with a maximum of 3 lines of salvage therapy (3rd salvage permitted in age-appropriate patients achieving at least 1 year remission from 2nd line salvage). In order to address uncertainty of model input variables, a probabilistic analysis in which model inputs were represented by probability distributions was utilized, permitting a Monte Carlo simulation with 5000 replications. Costs and utilities were discounted at a rate of 5% per annum. Subgroup analyses for the following iNHL histologies were performed using individualized parametric survival curves: follicular lymphoma (FL), mantle cell lymphoma (MCL), marginal zone lymphoma (MZL), lymphoplasmacytic lymphoma (LPL).
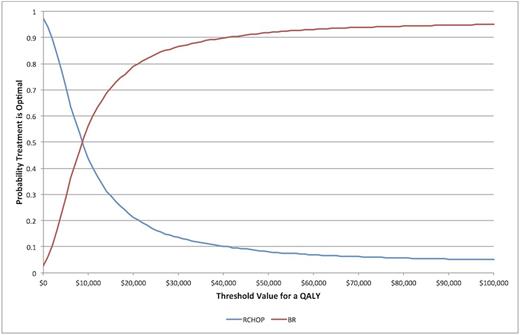
Results: The average costs and QALYs for the two treatment strategies were as follows: $116,811 and 5.86 QALYs for RCHOP; $121,364 and 6.38 QALYs for BR. The incremental cost per QALY gained for using BR with respect to RCHOP was $8,812 (Figure 1). Subgroup analyses revealed robust ICUR results: $27,398 (FL), $8,924 (MCL), $10,012 (MZL), $6,565 (LPL). For the commonly accepted willingness to pay threshold (WTP) of $50,000 per QALY, BR was the more cost-effective strategy 92% of the time in the entire cohort (Figure 2). In the subgroup analyses, BR was the more cost-effective strategy 66%, 82%, 64%, 86% of the time in FL, MCL, MZL, LPL respectively. ICUR results were robust to sensitivity analyses of key variables including age at study entry, maximum allowable age for therapy, duration of AEs, probability of death from palliation state and discount rate.
Conclusion: Our model suggests that BR is a cost-effective strategy for the frontline treatment of patients with iNHL as compared with RCHOP. The cost-effectiveness of BR may be driven by the upfront PFS advantage despite higher acquisition costs and is consistent in various iNHL histology subgroups. Our analysis supports the use of frontline BR for iNHL in the Canadian setting.
Cost-effectiveness acceptability curve
Incremental cost-effectiveness of BR relative to RCHOP with WTP threshold of $50,000 per QALY
Incremental cost-effectiveness of BR relative to RCHOP with WTP threshold of $50,000 per QALY
Bence-Bruckler:Lundbeck: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal