Abstract
Introduction: Systemic evaluation of toxicities after allogeneic hematopoietic stem cell transplantation (allo-HCT) has been limited. Ex vivo CD 34+ selection prior to allo-HCT reduces GVHD without increasing relapse, but is usually done in the context of myeloablative conditioning. Comprehensive collection of toxicities is particularly important in older patients (pts) who usually receive a reduced intensity conditioning.
Aim: To identify toxicity patterns in older pts and their association with overall survival (OS) and non-relapse mortality (NRM).
Methods: A retrospective analysis was performed at Memorial Sloan Kettering Cancer Center (MSKCC) including 200 pts who underwent CD34+ selection allo-HCT using the ClinicMACS® system between 2006-2012. All grade 3-5 toxicities by CTCAE v4.0 were collected and compared between pts ≥ 60 or <60yrs. Individual toxicities were organized into 91 toxicity categories and further into 17 organ-based groups. One toxicity per group per specified time period was used for statistical analyses. OS and NRM were calculated using Kaplan Meier methodology, and Cox regression was used for univariate analysis of risk of toxicities and survival outcomes across patient and treatment characteristics.
Results:
Eighty pts ≥ 60yrs with a median age of 64 (range 60-73) were compared to 120 pts <60yrs (Table 1). Busulfan, fludarabine, and melphalan conditioning with rabbit ATG was used in 72% of pts. Pts <60 were more likely to receive total body irradiation (45 vs 3%, p<0.001). Median follow-up in survivors was 48.2 months.
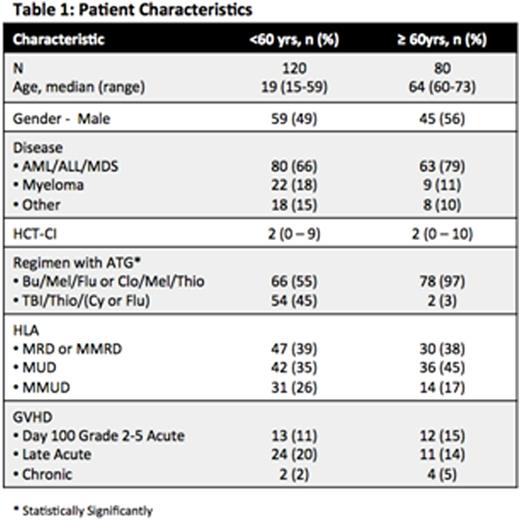
OS was not different between ≥60 and <60, with a 1-yr OS 70% vs 78% (p=0.07), respectively. NRM at 1-yr was also similar, 23% vs 13% (p=0.38), respectively (Figure 1A).
In pts ≥60, the most common toxicities by day 100 were metabolic with a cumulative incidence of 88% (95% CI 78-93%), infectious 84% (73-90%), hematologic 80% (69-87%), oral/gastrointestinal (GI) 48% (36-58%), cardiovascular (CV) 35% (25-46%), and hepatic 25% (16-35%). Pts ≥60 had a higher risk of neurologic [HR 2.63 (1.45 to 4.78), p=0.001] and CV [HR 1.65 (1.04 to 2.63), p=0.03] toxicities, but lower risk of oral/GI [HR 0.58 (0.41 to 0.83), p=0.003] compared to <60.
Univariate analysis of baseline characteristics identified associations with the most common toxicities in the first year. In pts ≥60, metabolic toxicity was increased if the donor was CMV seropositive [HR 1.9 (1.2-3.1), p=0.01], and decreased with albumin ≥ 4 [HR 0.6 (0.4-0.9), p=0.02] and an unrelated donor [HR 0.5 (0.3-0.9), p=0.01]. Diagnosis of multiple myeloma (MM) was associated with increased hematologic toxicity [HR 3.04 (1.4-6.4), p<0.001]. Risk of oral/GI toxicity was increased with patient CMV seropositivity [HR 2 (1.1-3.9), p=0.03] and decreased in males [HR 0.5 (0.3-0.9), p=0.02]. Both ferritin >1000 [HR 3.5 (1.5-7.9), p=0.002)] and patient CMV seropositivity [HR 2.8 (1.3-6.3), p=0.01] increased the risk of hepatic complications. No baseline characteristics were significantly associated with infectious or cardiovascular toxicities.
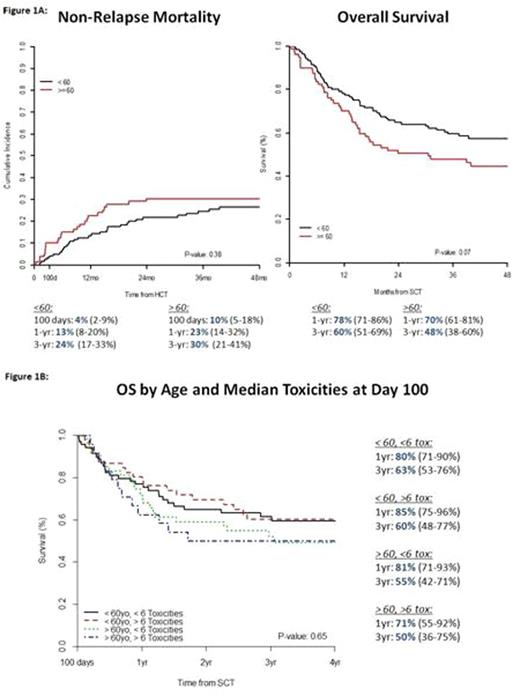
The median number of toxicities at Day 100 was 6. OS was not significantly different in pts ≥60 with ≥ median toxicities compared to pts with < median toxicities, and when compared to pts <60 yrs (p=0.65) (Figure 1B).
In further univariate analysis in pts ≥ 60 including baseline characteristics and the 17 toxicity groups, diagnosis of MM was the only baseline characteristic associated with worse OS (HR 2.4, p=0.04). NRM was increased with each year post age >60 (HR 1.2, p=0.004), the need to adjust busulfan dose lower (HR 3.1, p=0.03), patient CMV seropositivity (HR 2.7, p=0.04), and an HCT-CI ≥3 (HR 5.19, p=0.016). CV, endocrine, hepatic, neurologic, pulmonary, and renal toxicities were associated with worse OS, while CV, hepatic, neurologic, pulmonary, renal, and skin toxicities increased the risk of NRM (Table 2).
Conclusions: Older pts had similar OS and NRM when compared to younger pts, and the toxicity of a more intense regimen is potentially balanced by the absence of toxicity related to methotrexate and calcineurin inhibitors. While the median number of toxicities was similar between younger and older pts and to unmanipulated grafts based on reports of the BMT CTN, there was a difference in the type of toxicities by age. This is the first comprehensive toxicities assessment post CD34+ selected allo-HCT, and a prospective exploration of CD34+ selection in older pts is essential.
Koehne:Atara Biotherapeutics: Consultancy. Perales:Incyte Corporation: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Seattle Genetics: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Merck: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal