Abstract
Autoimmune hemolytic anemia (AIHA) is an antibody-mediated condition where the mainstays of treatment are corticosteroids and immunosuppressive therapies. This leads to substantial toxicity in patients with refractory or chronic disease and thus there is a need for improved therapies. BTK is a critical kinase in the B-cell receptor signaling cascade and we hypothesized that inhibition would decrease autoantibody responses. Supporting this, CLL patients taking ibrutinib, a first generation BTK inhibitor, experience very low rates of AIHA (Leukemia 2016). Based on this rationale we evaluated two BTK inhibitors, ibrutinib (ibr) and acalabrutinib (acal), in mouse models of AIHA to validate BTK as a target in this disease.
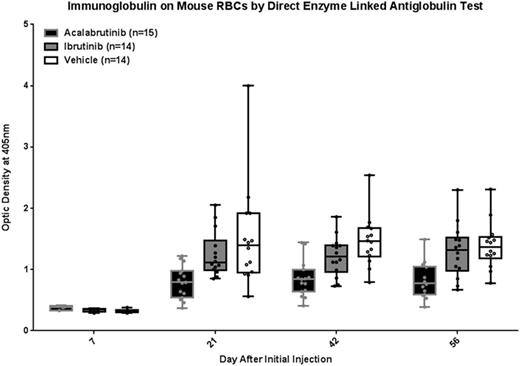
Young C3H/B6 F1 mice were treated with acal, ibr, or vehicle (veh) via drinking water for 2 weeks then injected intraperitoneally with washed rat red blood cells (RBCs) weekly for 4 weeks using the method of Playfair and Marshall-Clarke (Nature New Biology 1973) to induce a response against autologous RBCs starting 14 days after initial injection and peaking days 28-35. Drug treatment was continued throughout the study and using this delivery method BTK occupancy was 99.1% for acal, 96.1% for ibr, and 0% for veh. Quantitation of immunoglobulin on mouse RBCs was performed by a direct enzyme-linked anti-globulin test (DELAT) and by flow cytometry for surface immunoglobulin as a confirmatory method. Results from DELAT are shown in figure 1. A repeated measures model was applied to log-transformed data. On day 7, prior to autoimmune response, there were no statistically significant differences between treatment groups. However, on day 21 significantly less autoantibody was detected on RBCs in the acal group vs. both veh (p=0.001) and ibr (p=0.008); the ibr group showed a trend toward less autoantibody compared to veh but this did not reach statistical significance (p=0.542). This relationship between treatments held true at days 42 and 56 (acal vs. vehicle, p=<0.001 and p=0.001; acal vs. ibr, p=0.008 and p=0.004; ibr vs. veh, p=0.542 and p=0.542). These results were confirmed by flow cytometry. Hematocrit tests were performed by capillary tube centrifugation with no mice developing anemia and no differences between treatment groups excluding rapid RBC turnover as a cause of reduced immunoglobulin on RBCs.
As ibrutinib has been shown to alter macrophage function, we used a passive model to determine if BTK inhibitors changed clearance of antibody coated RBCs from circulation. After two weeks of treatment with acal (n=13), ibr (n=12), or veh (n=12) mice were injected with a single dose of anti-RBC antibody (anti-ter-119) and hematocrit was measured daily for 7 days. Median hematocrit values at baseline for the three groups were 53.7 (SD=1.3), 52.8 (SD=1.4), and 52.8 (SD=0.9) for acal, ibr, and veh respectively and fell to a low of 18.4 (SD=2.7), 18.0 (SD=3.2), and 15.8 (SD=1.9) on day 3 before recovering. A repeated measures model was applied to the data and no significant differences between drug treatment groups were found (p=0.177), indicating no effect of BTK inhibition on RBC clearance.
To examine anti-rat RBC antibody response washed rat RBCs were incubated with mouse plasma and analyzed by flow cytometry. Treatment groups were compared at day 42 after initial rat RBC injection. An analysis of variance (ANOVA) model was applied to log-transformed data. There was significantly less anti-rat RBC antibody in the acal group vs veh (p=0.008). In addition, there was decreased, albeit not statistically significant, antibody production with ibr compared to veh and acal compared to ibr (p=0.193 and p=0.102, respectively). This is the same trend as for autoantibody production and argues that BTK inhibition may reduce antibody responses.
In summary, treatment with acalabrutinib significantly reduced anti-RBC autoantibody response in our model compared to vehicle and ibrutinib, and autoantibody was consistently decreased in ibrutinib treated mice compared to vehicle although this did not reach statistical significance. The differences between inhibitors may be due to potency of BTK inhibition or effects on other targets of ibrutinib which is a less specific drug. This mechanism is under active investigation. These results support BTK as a potential therapeutic target in AIHA and the further development of acalabrutinib for this indication.
Woyach:Karyopharm: Research Funding; Morphosys: Research Funding; Acerta: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal