Abstract
Background: Sickle cell disease (SCD) is characterized by continuous oxidative stress through various mechanisms contributing to the pathophysiology and clinical course of sickle cell crises (SCC) and organ damage. L-glutamine is a precursor for the synthesis of essential metabolic redox cofactors including Nicotinamide Adenine Dinucleotide (NAD). Early studies have demonstrated that altered erythrocyte NAD redox potential was improved by oral L-glutamine therapy, suggesting that higher L-glutamine utilization in SCD exceeded de novo synthesis and its depletion played a role in oxidative stress.
A Phase 2 study of Pharmaceutical Grade L-glutamine (L-glutamine) versus placebo showed promising results on clinical endpoints. In a Phase 3 study with 230 SCD patients, randomized 2:1 to L-glutamine or placebo, the median number of SCCs was 25% lower for L-glutamine than placebo (L-glutamine 3.0 vs. placebo 4.0). An analysis of SCC events showed significant differences between groups (p < 0.01). Categories for pre-specified subgroups for Age, Gender and Hydroxyurea (HU) use were defined in the statistical plan. HU use was also a randomization stratification factor since HU is the only FDA-approved drug for SCD treatment. Examination of the HU subgroup for differences in L-glutamine effect across categories (HU vs. no HU) was appropriate since approximately 66% of patients remained on HU throughout the Phase 3 study. To investigate the subgroup treatment effect size, treatment by subgroup interaction and consistency of the primary endpoint across the categories of the subgroups, an examination was performed following the guidelines for reporting subgroup analysis.
Methods: The Negative Binomial Regression (NBR) model was utilized to generate an estimate of treatment effect and treatment by subgroup interactions of the three pre-specified subgroups. SCC events are most accurately described as counts and NBR is specifically intended for modeling count variables, usually for over-dispersed counts as seen in SCD studies. Rates of SCC for each treatment arm in a subgroup (Rate L-glutamine per 48 weeks / Rate placebo per 48 weeks) provide rate ratios, and an estimate of effect size, with 95% confidence intervals. A rate ratio <1.0 favored L-glutamine. Tests for treatment by subgroup interactions were conducted simultaneously. A significant interaction could be seen if the treatment is better than placebo for one category and worse than placebo for the other. Predefined categories for subgroups with corresponding sample sizes were as follows: Age: 5 - 18 years (n = 118) and ≥ 18 years (n = 111); Gender: Females (n = 124) and Males (n = 105). HU use before and during study: Yes (n = 153) and No (n = 76). Data for this Phase 3 subgroup report were obtained from an integrated dataset and the NBR model with treatment, subgroup, region and HU use as main effects and a treatment by subgroup interaction term was run with log (time on study) as an offset.
Results:
Age Subgroup:Both the 5 - 18 years and the > 18 years groups had a lower rate of SCCs per 48 weeks in the L-glutamine arm relative to placebo. The rate ratio was 0.93 [0.67 - 1.29] for the 5 - 18 years group and 0.64 [0.45 - 0.89] in the > 18 years group. There was no treatment by Age Group interaction (p = 0.118).
Gender Subgroup: Both the Female and the Male groups had a lower rate of SCCs per 48 weeks in the L-glutamine treatment arm. The rate ratio was 0.81 [0.59 -1.12] for the Female group and 0.73 [0.51 - 1.05] for the Male group, indicating consistent treatment effects across genders. There was no treatment by Gender interaction (p = 0.683).
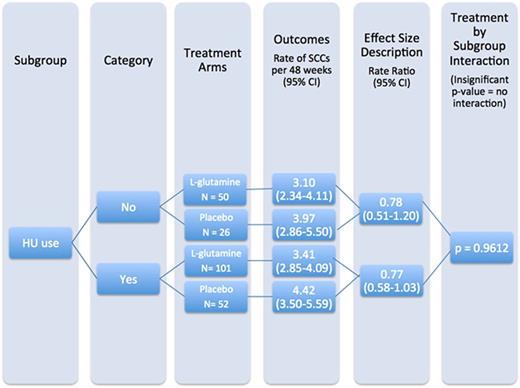
Hydroxyurea Use Subgroup: Both HU groups had a lower rate of SCCs per 48 weeks in the L-glutamine arm. The treatment effects were consistent across groups, with a rate ratio of 0.78 [0.51 - 1.20] for the group without HU use and 0.77 [0.58 - 1.03] for the group with HU use. There was no treatment by HU use interaction (p = 0.961). See Figure 1 for a graphic example.
Conclusion: For the Age and Gender subgroups, both categories in each subgroup benefited from L-glutamine but to varying degrees noted by the difference in rate ratios. For the HU use subgroup, both categories (on HU and not on HU) benefited from L-glutamine similarly. Lack of interactions in all subgroups indicated that regardless of category within a subgroup, treatment effect by Pharmaceutical Grade L-glutamine was beneficial.
Niihara:Emmaus Medical, Inc.: Employment, Equity Ownership. Viswanathan:Novartis: Speakers Bureau. Razon:Emmaus Medical, Inc.: Employment. Tran:Emmaus Medical, Inc.: Employment, Equity Ownership. Stark:Emmaus Medical, Inc.: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal