Abstract
Background: Venous thromboembolism (VTE) events are more frequent in neonates than in children of other age groups. The standard of care for pediatric anticoagulation includes unfractionated or low-molecular-weight heparin, or vitamin K antagonists. However, these treatments have limitations in terms of practicality of use in infants, such as parenteral administration, frequent monitoring and drug interactions. Alternative oral agents that address these shortcomings may improve compliance, efficacy and safety. The safety and efficacy of dabigatran etexilate (DE), a direct thrombin inhibitor, are established in adults; pharmacokinetic/pharmacodynamic (PK/PD) studies indicate linear PK. There is a relationship between dabigatran plasma concentration (PK) and its PD effects, resulting in reproducible dose-dependent prolongation in clotting times with rapid onset and offset of effect, which is consistent across populations. Although phase IIa studies suggest a similar PK/PD relationship of dabigatran in children and adults, the hemostatic system in infants differs from older children and adults, which may lead to a different PK/PD effect.
Objective: To demonstrate comparable PK/PD relationship of DE oral liquid formulation (OLF) between infants and older children and adults, and to assess safety and tolerability.
Methods: Open-label, multicenter, single-dose, single-arm, phase IIa pediatric study. Infants aged < 1 year diagnosed with VTE who completed standard anticoagulant treatment for VTE were enrolled. Exclusion criteria included < 37 weeks gestational age at birth, weight < 3 kg, major bleed with standard anticoagulant and swallowing abnormalities. Patients received DE OLF (based on age- and weight-adjusted nomogram) and were followed up for 30 (+7) days. The primary endpoints were PK/PD related, measured at 2 hrs and 12 (±2) hrs after DE administration. The PK endpoint was plasma concentration of total dabigatran, and PD endpoints were activated partial thromboplastin time (aPTT), ecarin clotting time (ECT) and diluted thrombin time (dTT). Secondary endpoints included incidence of all bleeding events and adverse events (AEs), acceptability and tolerability. Descriptive statistics were applied to the endpoints. PK/PD relationship was analyzed using a simple linear regression model and nonlinear Emax model to confirm whether models used in previous studies were consistent with the pediatric data of this study.
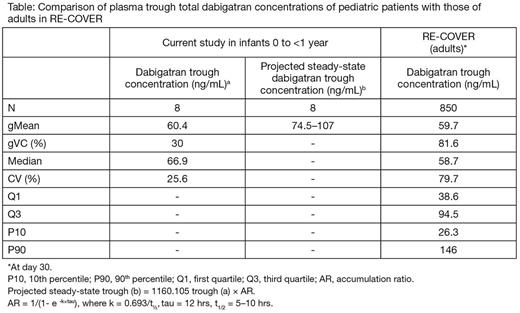
Results: Ten patients were screened and 8 entered the study (mean age [SD]: 89 [52] days; range: 41-169 days). Patients received a single dose of DE OLF. The geometric mean (gMean) total dabigatran plasma concentrations 2 hrs post dose (around peak concentrations) was 120 ng/mL with geometric coefficient of variation (gCV) of 62.1%, which indicated moderate variability. The gMean at 12 hrs post dosing was 60.4 ng/mL (gCV 30%), which indicated low variability. The projected steady-state dabigatran trough concentrations were largely comparable to those observed in adults with VTE (Table).
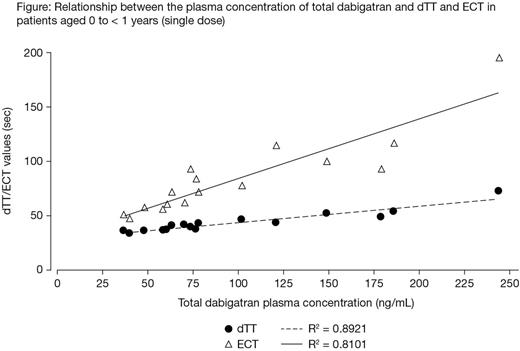
A linear PK/PD relationship was observed for ECT (ECT ratio) and dTT (dTT ratio) (Figure). The relationship between total dabigatran concentration and aPTT (aPTT ratio) was nonlinear. The observed PK/PD relationships were similar to those in adult and adolescent patients with VTE, except for patients aged < 2 months, in whom a slight upward shift of aPTT of 10-20% (average) and ECT by 10-15% was observed relative to adults. There were no AEs, deaths or treatment discontinuations during the treatment period. None of the treated patients had any bleeding events or thromboembolic events during the study. One patient had a serious AE (aortic stenosis) during the post-treatment period, which was not considered treatment related by the investigator. There were no clinically relevant or unexpected laboratory findings. The majority of patients were assessed with good tolerability (six patients, 75%), and one patient each (12.5%) was evaluated with satisfactory or bad tolerability.
Conclusion:
In this small population of infants (aged < 1 year), DE OLF was well tolerated without any treatment-related AEs, thromboembolic or bleeding events. The observed PK/PD relationships were consistent with the established profile in adult and adolescent patients with VTE.
Halton:Boehringer Ingelheim: Other: Pediatric Expert Working Group for Boehringer Ingelheim. Picard:Boehringer Ingelheim: Employment. Harper:Boehringer Ingelheim: Employment. Huang:Boehringer Ingelheim: Employment. Brueckmann:Boehringer Ingelheim: Employment. Gropper:Boehringer Ingelheim: Employment. Maas:Boehringer Ingelheim: Employment. Tartakovsky:Boehringer Ingelheim: Employment. Nurmeev:Boehringer Ingelheim: Other: Investigator fees. Mitchell:Boehringer Ingelheim: Consultancy; Pfizer: Consultancy; Bristol Myers Squibb: Consultancy. Albisetti:Boehringer Ingelheim: Other: Pediatric Expert Working Group.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal