Abstract
Background: Deficiency of plasma ADAMTS13 (a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13), a von Willebrand factor (VWF)-cleaving enzyme, in humans results in thrombotic thrombocytopenic purpura (TTP), a potentially fatal syndrome. Previous studies have demonstrated that ADAMTS13, particularly the C-terminal domains of ADAMTS13 may stimulate or inhibit angiogenesis, depending on the growth environment. However, the role of ADAMTS13 in vascular development in vivo is not known.
Objective: To identify the novel function of ADAMTS13 beyond the proteolysis of VWF, we generated ADAMTS13 knockdown and knockout zebrafish to determine the vascular development and propensity for development of thrombosis.
Methods: Morpholino anti-sense RNA was microinjected to the embryos of a double transgenic Tg (gata1:dsRed; fli1:eGFP)zebrafish to transiently block ADAMTS13 translation. Also, CRSIPR/Cas9 system was employed to generate ADAMTS13 knockout zebrafish. The vascular development and thrombus formation were determined by fluorescent microscopy.
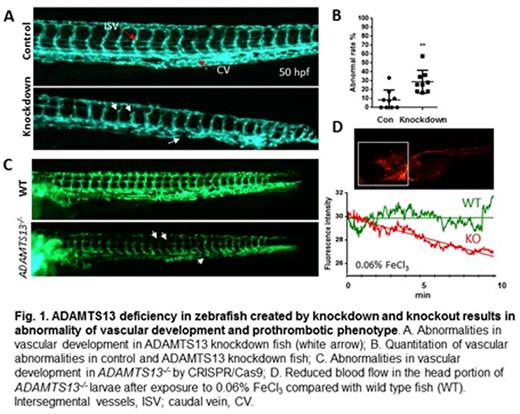
Results: Approximately 30% of ADAMTS13 knockdown zebrafish exhibited vascular abnormalities in the dwarf intersegmental vessels and narrow caudal vein plexus (Fig. 1A and B). To confirm the phenotype, ADAMTS13 knockout using CRSIPR/Cas system was performed. Approximately 85% of the F0 fish carried insertion or deletion mutations in the targeted region. The founder fish were outcrossed to wild type fish to generate heterozygous F1 fish. Fourteen different mutation sequences with germ line transmission were identified in 33 F1 mutants. The siblings carrying an 8-bp deletion mutation, which created a premature stop codon in the signal peptide region of ADAMTS13, were then crossed to produce F2 progeny. The genotypes and protein expression were further confirmed by sequencing and by Western blot, respectively. As shown, a significant higher rate of vascular development defects, particularly in intersegmental vessels and caudal vein, was observed in homozygous ADAMTS13 knockout fish compared to the heterozygous and wild type fish (Fig. 1C). Thrombosis was triggered by incubation of larvae with various low concentrations (0.06%-0.25%) of FeCl3 and determined by the real-time change of fluorescence intensity within 10 min in the head region of larvae under a fluorescent microscope. When compared with wild type fish, ADAMTS13-/- fish demonstrated dramatically decreased fluorescence signal (Fig.1D), indicative of the cessation of blood flow elsewhere in the body after FeCl3injury.
Conclusion: Our results demonstrate that severe deficiency of ADAMTS13 in zebrafish results in abnormalities of vascular development and increases the propensity of thrombosis after oxidative injury in fish larvae. These results suggest that ADAMTS13 may have other substrates than VWF. The zebrafish model may turn out to be a powerful tool for identifying a novel pro-angiogenic or anti-thrombotic agent.
Zheng:Alexion: Research Funding; Ablynx: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal