Abstract
Lung diseases are the second leading cause of death worldwide. There is a growing understanding that lung stem cells, rather than being distributed throughout the tissue, are concentrated in specialized niches. Thus, in our previous study we demonstrated that in analogy to bone marrow (BM) transplantation, lung progenitors could navigate through the blood to their appropriate niches, provided that the niches were cleared of endogenous cell populations (Nature Medicine 2015). Lung injury was induced by Naphthalene, and lung progenitors of all lineages were further depleted by 6GY total body irradiation (TBI) 48 hrs after Naphthalene exposure; at this time, endogenous lung progenitors proliferated extensively.
In the present study, we further established the feasibility of our approach in an allogeneic setting. To that end, we induced central immune tolerance towards donor cells, with no need for chronic immune suppression, by virtue of hematopoietic stem cells also present at high levels in fetallung alongside epithelial progenitors. The possibility that the E16 lung might contain high levels of hematopoietic progenitors was first suggested by examination of the peripheral blood of C57BL/6 mice transplanted with GFP+ E16 lung cells, or of RAG-SCID mice (H2Kb) receiving C3H (H2Kk) E16 lung cells. Robust chimerism was also documented in the BM and spleen of all transplanted mice.
Based on this initial observation, we attempted to define by FACS the level of putative hematopoietic progenitors in E16 lungs. We thus evaluated two commonly used phenotypes, namely, LSK (lineage-, SCA-1+, C-KIT+) and SLAM (lineage-, CD48-, CD150+). Indeed, we found a similar level of LSK and SLAM cells in the fetal liver and lung, representing about 20-40% of the levels found in the adult BM. A competitive chimerism assay in which normal adult BM cells from a CD45.1+ C57BL/6 donor compete with E16 lung cells from a GFP+CD45.2+ C57BL/6 donor, revealed marked capacity of the E16 lung cells to induce robust chimersim following infusion of a 1:1 mixture of these cells into lethally irradiated CD45.1+ C57BL/6 recipients. Thus, at 9 months post-transplant, 4/4 mice exhibited blood cells derived from the lung donor. In 2 out of 4 mice, levels ofchimerism were above 30%, strongly indicating the robust capacity of the lung hematopoietic progenitors for self-renewal. Based on our ability to induce durable hematopoietic chimeras in syngeneic recipients following transplantation of E16 lung cells, we next developed a sub-lethal transplantation protocol enablingchimerism induction of both non-hematopoeitic cells in the lung, and hematopoietic cells in the blood, liver, spleen and thymus ofmis-matched recipients.
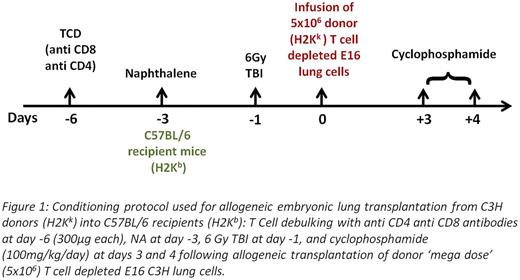
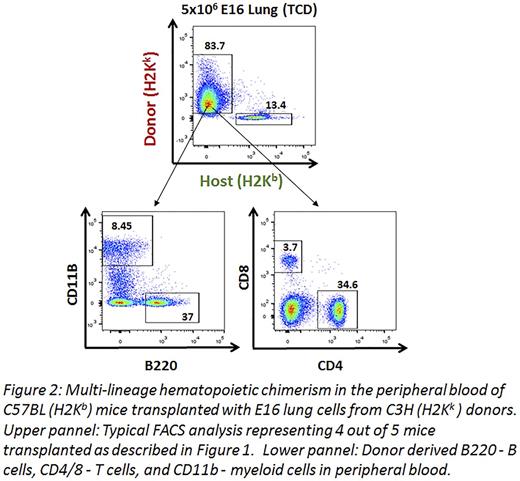
The protocol used was based on recent work in haploidenticalhematopoietic stem cell transplantation (HSCT), and comprised transient T cell debulkingof host CD4 and CD8 T cells, megadoseT cell depleted HSCT, and post-transplant cyclophosphamide (CY). This conditioning was coupled with NA, and 6GY TBI to vacate lung progenitor niches (Fig.1). T cells, already present at E16 lungs, were removed from the donor lung preparation by magnetic beads. Similar to our results in haploidenticalBMT we found that chimerisminduction required the use of a 'megadose' of stem cells (5x106 compared to 1x106 in the syngeneic model). When tested at 2 months post-transplant, 4 out of 5 mice exhibited substantial hematopoieticchimerism in the BM, liver, thymus, spleen and blood with multi lineage expression, including B cells (B220), T cells (CD4/CD8) and myeloid cells (CD11b) (Fig. 2).
Furthermore, 3 months post-transplant, donor-derived lung "patches" were present, exhibiting marked lungchimerism within both functional epithelial lineages (AEC1/2, marked by AQP5/SPC markers) and mesenchymal/endothelial lineages (marked byNestin/CD31 markers) (Fig. 3 a-d), confirming that the hematopoieticchimerisminduced tolerance towards donor non-hematopoietic lung cell lineages. The high level of hematopoietic progenitors with capacity for self-renewal in thefetallung, alongside non-hematopoietic progenitors, offers a novel approach for allogeneic stem cell transplantation without any need for chronic immune suppression. Further fine tuning is needed to replace NA with clinically approved agents and to define the minimal TBI dose required for effective conditioning.
*C.H.K and C.R. contributed equally
Hillel Karniel:Yeda LTD: Patents & Royalties. Rosen:Yeda LTD: Patents & Royalties. Bachar Lustig:Yeda LTD: Patents & Royalties. Shezen:Yeda LTD: Patents & Royalties. Reisner:Cell Source LTD: Consultancy, Equity Ownership, Patents & Royalties, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal