Abstract
Background
The prognosis for adults diagnosed with acute myeloid leukemia (AML) remains poor, with a five-year survival of 25%. This prognosis is even more dismal in older patients, and those who are not well enough to receive standard induction chemotherapy. Speaking to the need for new therapies is the fact that our therapeutic backbone - a combination of cytarabine and an anthracycline - remains unchanged for 40 years.
Differentiation therapy (e.g. ATRA and Arsenic trioxide) revolutionized care for the small subset of patients diagnosed with acute promyelocytic leukemia (APL). Where APL was once the worst form of myeloid leukemia, it now carries the best prognosis, with a five-year survival exceeding 85%. Replicating the success of differentiation therapy in the remaining 90% of patients with AML has remained elusive, and was the motivation behind our studies.
Hypothesis
AML comprises disparate genetic sub-types, but one shared hallmark is that the leukemic blasts are frozen at an immature and self-renewing stage of development. Despite AML's heterogeneity, 70% of cases overexpress HoxA9, a gene that must be downregulated in the process of normal myeloid cell differentiation. We hypothesized that the process through which HoxA9 maintains the undifferentiated state represented a commonly dysregulated node, and that therapies that bypass this node would be useful in the treatment of AML. Thus, we asked the question: 'can we identify small molecules that overcome myeloid differentiation arrest?'
A Phenotypic Differentiation Screen
We established a HoxA9-based AML screening system that permitted an unbiased flow cytometry phenotypic differentiation screen. We screened 330,000 small molecules for those with myeloid differentiation activity. Target identification of our lead compounds led to the unexpected discovery that inhibitors of dihydroorotate dehydrogenase (DHODH) can overcome myeloid differentiation arrest. DHODH is a mitochondrial enzyme involved in the endogenous synthesis of pyrimidines. The modulation of DHODH had not previously been shown to affect myeloid differentiation.
DHODH Inhibitors in the Treatment of Leukemia
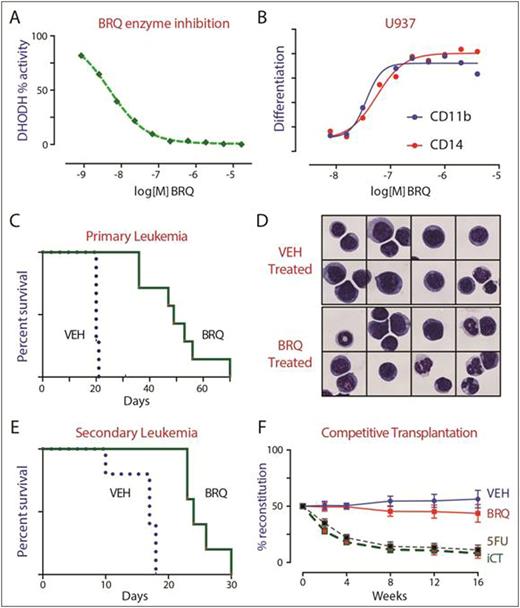
The small molecule brequinar is a potent DHODH inhibitor (Panel A). Brequinar triggered myeloid differentiation in vitro (Panel B) and in vivo. Brequinar was highly active in vivo, as demonstrated in syngeneic murine AML models (HoxA9+Meis1 and MLL/AF9) as well as xenotransplant AML models (THP1, HL60, MOLM13, OCI/AML3).
In an aggressive MLL/AF9 model of AML, treatment of mice with brequinar caused myeloid differentiation (Panel D), reduced leukemic cell burden, and improved overall survival (Panel C). Furthermore, treatment with brequinar reduced the number of leukemia stem cells, reduced colony-formation activity, and depleted the number of leukemia initiating cell activity (Panel E). These findings support the hypothesis that the myeloid differentiation resulting from DHODH inhibition is both phenotypic and functional, such that the matured leukemia cells lose their self-renewing capability.
DHODH Inhibitors and Normal Hematopoiesis
Treatment with brequinar was better-tolerated and more effective than treatment with cytotoxic chemotherapy. Unlike cytarabine and doxorubicin, brequinar could be given for many weeks without cumulative toxicity. To assay the effect of DHODH inhibition on normal cells, we performed competitive bone marrow transplantation assays. Mice were treated with brequinar, 5-fluorouracil or induction chemotherapy. Their bone marrow was transplanted in competition (1:1) with normal (untreated) bone marrow, to gauge the effect of therapy on hematopoietic stem cell (HSC) function. Treatment with 5-FU and induction chemotherapy led to a dramatic loss of HSC fitness. In contrast, HSCs from mice treated with brequinar were functionally equivalent to those of untreated mice (Panel F) supporting the notion that DHODH inhibition does not lead to detrimental differentiation of the HSC compartment.
Discussion
The mechanism for the selective vulnerability of leukemia cells to DHODH inhibition remains under investigation. Our studies point towards DHODH as a new metabolic target; DHODH inhibitors may provide differentiation therapy for patients with AML.
Sykes:Bayer Pharma AG: Research Funding. Meyer:Bayer Pharma AG: Employment. Stoeckigt:Bayer Pharma AG: Employment. Janzer:Bayer Pharma AG: Employment. Scadden:GlaxoSmithKline: Research Funding; Dr. Reddy's: Consultancy; Fate Therapeutics: Consultancy, Equity Ownership, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties; Teva: Consultancy; Apotex: Consultancy; Bone Therapeutics: Consultancy; Magenta Therapeutics: Consultancy, Equity Ownership, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal