Abstract
Title: Collection and Processing Considerations in Haploidentical Stem Cell Transplantation Utilizing a 2-Step Methodology
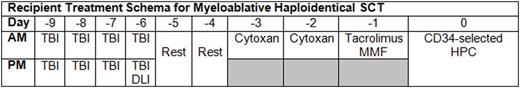
Background: Haploidentical transplantation is an active area of research because only about one-third of patients have an HLA-matched sibling and searching for an HLA-matched-unrelated donor can delay urgent need for alloBMT. However, nearly all patients have HLA-haploidentical related donors. The incidence of graft rejection, infection, and GVHD has been minimized by improving conditioning regimens and the use of cyclophosphamide-based T-cell tolerization. There are unique collection and processing considerations associated with such transplants. We describe the unique collection considerations for the Thomas Jefferson University 2 Step regimen (TJU2) (Grosso D, Blood 2011), where the donor lymphocyte infusion (DLI) precedes the CD34+ selected hematopoietic progenitor cell (HPC) transplant.
The DLI target dose in this study is fixed at 2 x 10e8 CD3+/recipient kg. The HPC collection is CD34+ cell selected. The post-selection target cell doses/recipient kg are 2-10 x10e6 CD34+ cells and < 5 x 10e4 CD3+ cells. The points to consider in determining donor blood volume to be processed during apheresis collection are:
1. Although 0.14 x 10e8 CD3+/L processed/recipient kg is a median CD3+ dose collected with G-CSF mobilization (Korbling, JCA 2001), where the absolute lymphocyte count increases ~2-fold above its baseline (Holig, Blood 2009, Anderlini Transfusion 1996), the median dose for a non-mobilized collection is 0.07 x 10e8 CD3+/L processed/recipient kg (Korbling, JCA 2001). Therefore large volume DLI collection may need to be performed.
2. The collection facility's lymphocyte or CD3+ collection efficiency. Donor pre-collection peripheral blood CD3+ count is typically not available.
3. The collection facility's CD34+ cell collection efficiency. This can be calculated because the donor's peripheral blood CD34+ cell count is typically available.
4. Higher CD34+ cell doses appear to be beneficial in haploidentical transplant (Reisner, Blood 2011). Therefore we designed the HPC collection volume to obtain a post-CD34+ cell selection target dose of 7 x 10e6/recipient kg.
5. The processing facility's CD34+cell selection recovery. This typically varies from 30-100%.
Methods: All procedures except one DLI (performed with the Spectra Optia CMNC) were performed using the Cobe Spectra MNC procedure (Terumo Corp). The following algorithms were used to estimate the donor blood volume ideally to be processed to ensure that target doses were reached:
DLI: [(recipient kg x CD3 target/kg) / (0.6 x ALC)] / (median lymphocyte CE)
0.6 is the lower limit of a normal percentage of CD3+ lymphocytes
ALC = donor absolute lymphocyte concentration on the day of collection
CE = collection efficiency (facility-specific median = 0.58)
HPC: [(recipient kg x post-selection CD34 target/kg) / PB CD34+] / (median CD34 CE) / (minimum CD34 selection recovery)
Post-selection CD34 target/kg = 7 x 10e6/kg
PB CD34+ = donor peripheral blood CD34+ concentration on day of collection
CE = collection efficiency (facility-specific median = 0.45)
Facility-specific minimal CD34+ selection recovery=0.31
CD 34+ cell enriched products were obtained by incubation of mobilized HPC, Apheresis products with Miltenyi CD34+ reagent and subsequent positive selection on the CliniMACS Plus (Milteyi Biotec GmbH) according to manufacturer's specifications.
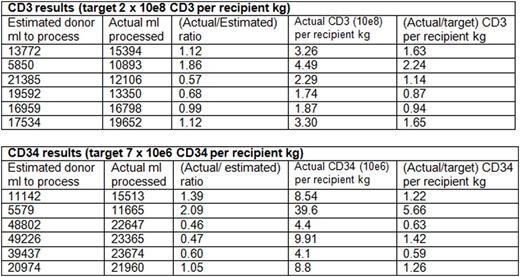
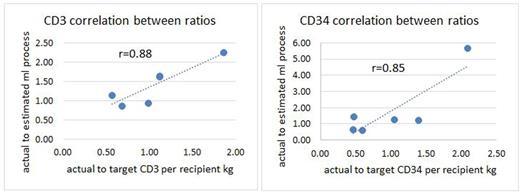
Results: Our results in 6 donor-recipient pairs were the following:
Discussion: The variability in the predictive value is due to variabilities in lymphocyte CE (range 0.38-0.94), CD34+ CE (range 0.36-0.67), and CD34+ selection recovery (range 0.31-0.81). Nevertheless, the correlation coefficients are high and as the ranges of CEs and CD34+ selection recovery are optimized and narrowed, the predictive value of these formulas will increase. Of note, all collections except one were performed with the Cobe Spectra MNC procedure. With the transition to the Optia CMNC procedure, where the collection interface is controlled by the device, the CEs are expected to become more consistent.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal