Abstract
The PF4-dependent P-Selectin Expression Assay (PEA) is a novel assay for diagnosis of heparin-induced thrombocytopenia (HIT) which, in preliminary testing, demonstrates higher diagnostic accuracy than the "gold standard" serotonin release assay (SRA) (Padmanabhan et al. Chest, 2016 epub). Here, we describe two cases of severe thrombotic HIT in which the PEA detected pathogenic HIT antibodies (abs) before the SRA tested positive, and in one case, earlier than the highly sensitive PF4 ELISA. These findings have implications for diagnosis and monitoring of the response to therapy in HIT.
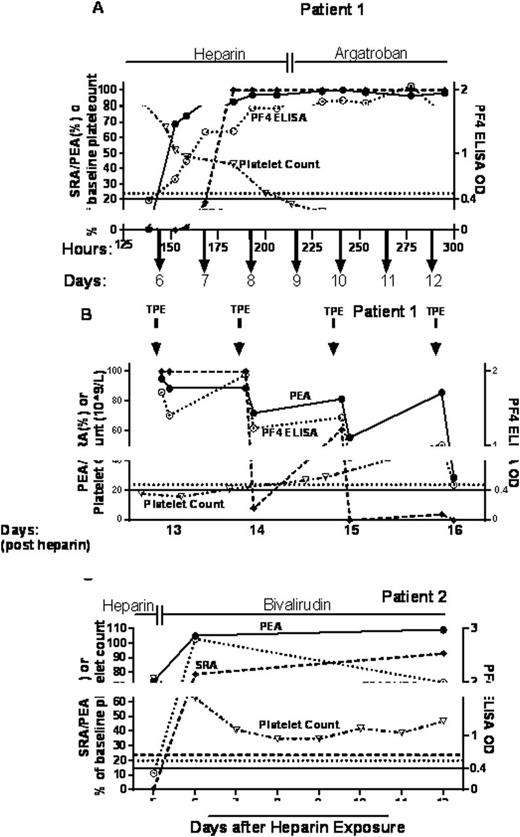
Patient 1, a 67 yr old woman was admitted for treatment of a Bochdalek hernia and was started on unfractionated heparin (UFH). Her course was complicated by a declining platelet count and on the eighth day of admission PF4 ELISA and SRA tests were strongly positive (Fig 1A). Vascular imaging demonstrated venous thrombosis in both upper and lower extremities. Pre- and post-diagnosis patient samples were tested in the PF4 ELISA, SRA and PEA (Fig 1A). The PEA was positive at 152 hrs (~6 days) post-heparin exposure (69% expression), but the PF4 ELISA was only weakly positive (OD 0.67) and the SRA was negative (0%) at a time when the platelet count was 52% of baseline (Fig 1A). The SRA was still negative (18%) 16 hours later (168 hrs). Thirty hours after the first positive PEA test result, the SRA became positive (100% release). The patient's condition was then further complicated by sepsis-associated multisystem organ failure (MSOF) and therapeutic plasma exchange (TPE) was instituted. TPE treatment led to a rapid reduction in the SRA but only a gradual decline in PEA and ELISA test results (Fig 1B), in line with gradual improvement in platelet counts. Dissociation between SRA and PF4 ELISA test results following TPE has been described previously (Blood. 2015;125:195-8). In our patient, a PEA-SRA dissociation was also observed. Early in this patient's course, PEA-positive/SRA-negative abs were clearly "pathogenic," causing a significant decrease in platelet counts. Thus, it is plausible that PEA-positive/SRA-negative abs detected during the course of TPE were also "pathogenic". PEA results during the course of TPE appeared to correlate better with clinical status in light of the gradual improvement in platelet counts. Unfortunately, the patient expired after the 4th TPE secondary to complications of MSOF.
Patient 2, a 57 yr old man was admitted for a creation of a thigh flap in preparation for knee replacement. Postoperatively, the flap became pale with poor pulses and UFH was started. On day 5 platelets dropped 25% from baseline and PF4 ELISA and SRA were both negative (Fig 1C). A strong suspicion of HIT prompted repeat testing on day 6, and yielded positive SRA (79%) and PF4 ELISA (2.8 OD) test results. Notably, the PEA was positive (74%) on Day 5 when both PF4 ELISA and SRA were negative. All tests remained strongly positive through day 12. Thrombocytopenia persisted (Fig 1C) and extensive venous thrombosis of the right upper extremity developed on day 15. The patient was transitioned to Coumadin after platelet recovery and discharged on Day 30.
Previous studies indicate that the PEA is at least as accurate as the SRA for detection of pathogenic abs (Padmanabhan et al Thromb Haemost. 2015;114:1322-3, Padmanabhan et al. Chest, 2016 epub). Experience with these two cases suggests that the PEA may in some cases detect pathogenic antibodies before the SRA becomes positive and may be superior to the SRA for monitoring response to treatment. Because of its technical simplicity, the PEA has the potential to be performed on demand, rather than through a referral laboratory, facilitating early diagnosis and treatment.
Inverted open triangles, closed circle, open circle and closed diamonds depict platelet count, PEA, PF4 ELISA and SRA, respectively. Horizontal lines indicate SRA, PEA and PF4 ELISA positive cut-offs of 20%, 24% and 0.4 OD, respectively.
Inverted open triangles, closed circle, open circle and closed diamonds depict platelet count, PEA, PF4 ELISA and SRA, respectively. Horizontal lines indicate SRA, PEA and PF4 ELISA positive cut-offs of 20%, 24% and 0.4 OD, respectively.
Padmanabhan:Fenwal (Fresenius Kabi): Research Funding; Schlesinger & Associates: Consultancy, Honoraria; LEK Consulting: Consultancy, Honoraria; Mallinckrodt Pharmaceuticals: Consultancy, Honoraria; Terumo BCT: Consultancy, Honoraria; Bloodcenter of Wisconsin: Patents & Royalties: A patent application has been filed on a Method of detecting platelet activating antibodies that cause heparin-induced thrombocytopenia/thrombosis; PCT/US14/62591. Jones:Bloodcenter of Wisconsin: Patents & Royalties: A patent application has been filed on a Method of detecting platelet activating antibodies that cause heparin-induced thrombocytopenia/thrombosis; PCT/US14/62591. Bougie:Bloodcenter of Wisconsin: Patents & Royalties: A patent application has been filed on a Method of detecting platelet activating antibodies that cause heparin-induced thrombocytopenia/thrombosis; PCT/US14/62591. Aster:Bloodcenter of Wisconsin: Patents & Royalties: A patent application has been filed on a Method of detecting platelet activating antibodies that cause heparin-induced thrombocytopenia/thrombosis; PCT/US14/62591.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal