Abstract
Introduction
Langerhans cell histiocytosis (LCH) is the most common histiocytic disease in humans with a complex pathophysiology and unknown etiology. LCH has been recently reclassified from an inflammatory disease to a neoplastic disorder due to the high prevalence of mutations in the MAP kinase pathway. Recurrent mutation in BRAF kinase (BRAFV600E) was detected in more than 50% of patients. We recently showed that BRAFV600E mutation can be detected in the circulating CD34+ cells in LCH patients and that the expression of the mutation in distinct hematopoietic compartments defines the stage and the clinical risk in LCH disease (Berres et al. J Exp Med 2014). Knowledge on induction and progression of LCH is limited due to appropriate models resembling the human disease. We recently generated so called MISTRG mice where the mouse cytokines M-CSF, IL-3, GM-CSF, and thrombopoietin were replaced by their human variants and that in addition express a human transgene of SIRPa (Rongvaux et al. Nat Biotech 2014). These mice efficiently support human myelopoiesis from human hematopoietic stem and progenitor cells (HSPCs). We here tested if xeno-transplantation of BRAFV600E transduced human CD34+ cells in these mice would result in LCH.
Materials and Methods
Human CD34+ HSPCs obtained from healthy donor cord blood samples were lentivirally transduced using a bidirectional promoter vector harboring BRAFV600E and GFP (as a marker for transduction efficiency) in the presence of supportive growth factors. As control, BRAFV600E construct was substituted by luciferase gene. The In vivo experiments were performed by injection of transduced CD34+ into newborn (n=10, of which 5 control and BRAFV600E respectively) and adult (n=8, of which 4 control and BRAFV600E respectively) MISTRG mice, which were sublethally irradiated prior to transplantation. Mice were sacrificed once symptoms of sickness were observed and analyzed using flow cytometry and immunohistochemistry staining for investigating the development of LCH-like disease. The influence of BRAFV600E mutation on proliferation and lineage commitment of human HSPCs was investigated using colony forming unit (CFU) assay and short term liquid cultures. For CFU assays, the transduced human CD34+ cells were plated in M4230 medium in the presence of supportive growth factors. After 14-16 days the obtained colonies were scored microscopically and measured by flow cytometry for further characterization of the cells. For short term liquid cultures, transduced cells were kept in culture in the presence of supportive growth factors and measured by flow cytometry for expression of human myeloid and lymphoid markers.
Results
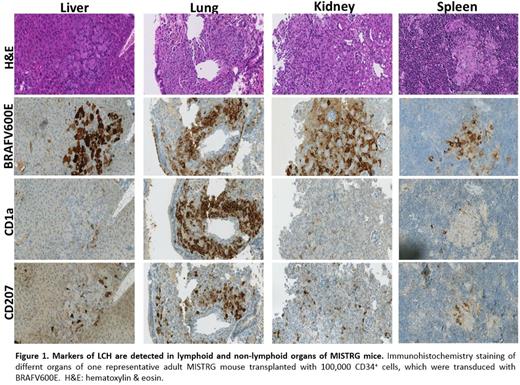
Mice transplanted with human CD34+ HSPCs expressing BRAFV600E developed anemia and reduced WBC and RBC counts. Analysis of lymphoid and non-lymphoid organs demonstrated the accumulation of ''atypical'' BRAFV600E expressing dendritic cells in granuloma-like lesions. Using immunohistochemistry staining we observed the expression of LCH-associated markers, CD1a and CD207 to a varying extent in different organs (Figure 1). We did not observe development of hairy cell leukemia. The in vitro studies revealed that the expression of BRAFV600E blocks the commitment of erythroid progeny toward mature erythrocytes shown by lower number of BFU-E colony type and reduced CD235ahigh-expressing cells. BRAFV600E seemed to increase monocytosis and generation of classical dendritic cells in short term liquid cultures, without affecting the proliferation potential of human HSPCs.
Conclusions and outlook
We demonstrate that expression of BRAFV600E in human HSPCs induces LCH-like disease in MISTRG mice. The involvement of wide range of organs resembles high-risk LCH in humans and confirms our already reported data with aggressive LCH being a HSPC disease. Using this model system will further support basic understanding of LCH patho-biology and testing of targeted therapies in LCH.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal