Abstract
Background: APL is a highly curable malignancy with reported survival above 90% in many co-operative group studies. However these spectacular results are not evident in the general population. US SEER data and other population based studies from Swedish Cancer Registry and Brazil showed that early deaths (ED) can be as high as 30%, leading to a considerably lower survival compared to clinical trials where ED is around 5-10%. Decreasing ED remains a global challenge and the highest priority at all leukemia treatment centers and will result in population wide survival in this most curable leukemia. We report results of our prospective trial using a set of simplified treatment guidelines along with expert support designed to decrease ED.
Methods: A network of leukemia treatment centers was established in Georgia, South Carolina and neighboring states. An aggressive outreach effort was made by visiting most of the leukemia treatment centers to publicize the concept and educate treating physicians in the community about ED in APL. The protocol provides a simplified two page treatment algorithm that emphasizes quick diagnosis, prompt initiation of therapy and proactive and aggressive management of the major causes of death during induction. Expert and treating physician communication was established very early when a diagnosis of APL was suspected and was maintained until the completion of induction. Study was approved by local IRBs (if applicable) and funded by the Lymphoma Leukemia Society (LLS). Informed consent was obtained upon confirmation of a diagnosis of APL and there were no exclusion criteria. Patient accrual was initiated in July 2013 and continued till May 2016 when the accrual goal of 120 was met on an intent to treat basis. Statistics are descriptive.
Results: Between 7/2013 and 5/2016, 120 patients were enrolled at 5 large leukemia centers (n=54, 45%) and 24 community hospitals (n=66, 55%). Only 3 hospitals treated more than 3 APL patients/year. Median age was 54 years (range 21-84 years). 68 were male. 84% were low risk (WBC < 10,000/mm3) and median WBC count was 4.3 (range 0.3-170,000/mm3). ATRA was initiated at suspicion of APL diagnosis in 100% of patients and was the only treatment in 2(1.5%) patients. Arsenic was combined with ATRA in 93 (81.5%) patients while the other 17% received chemotherapy. 15(13%) had bleeding complications at presentation. Treatment course was complicated by infection and differentiation syndrome (DS) in 31(28%) and 40(34%) patients respectively.
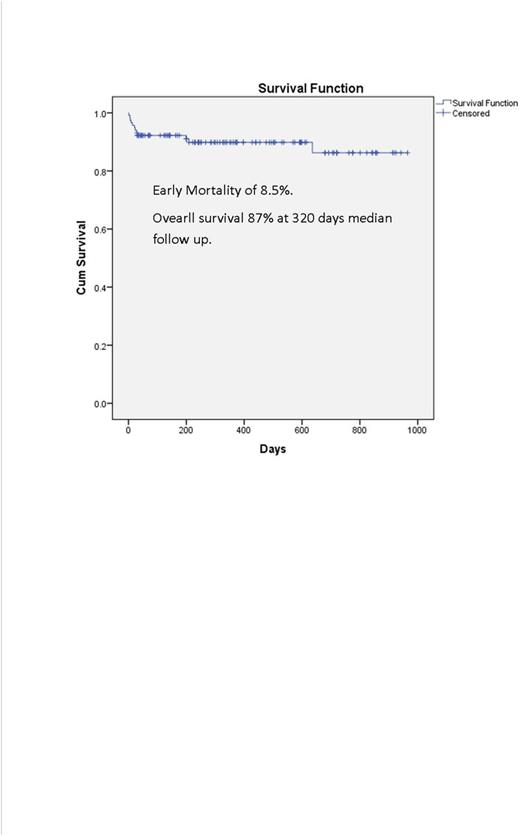
There were 12 early deaths, of which 1 was a Jehovah's Witness who declined transfusions and 1 who enrolled 12 days after diagnosis while in multi-organ failure. Incidence of ED was 10/118 (8.5%). The cause of death was disseminated intravascular coagulation (DIC) (n=4), DS (n=2), infection (n=1), anemia (n=1), multi-organ failure (n=4). With a median follow-up of 10.6 months, 2 low risk patients relapsed: I due to non-compliance 1 year after diagnosis and 1 with CNS relapse 3 months after completing consolidation. With a median follow up of 320 days (range 1-965) overall survival (Figure 1) was 87%. There were four late deaths; relapse (n=1), second cancer (n=1) and non-APL related comorbidities (n=2).
Conclusions: Results of this prospective trial showed that a simplified treatment algorithm along with support from experts and co-management with treating physicians in the community decreased induction mortality (8.5%) and improved survival (87%) compared to SEER data (1 year relative survival of 71%). We believe our experience warrants large scale implementation and is presently approved as an ECOG/ACRIN trial (EA9131). This model can be applicable to other cancers and life-threatening diseases.
Jillella:Leukemia Lymphoma Society: Research Funding. Heffner:AbbVie: Research Funding; Pharmacyclics: Research Funding; Celgene: Research Funding; Millennium: Research Funding. Stuart:Astellas: Research Funding; Celator: Research Funding; Sunesis: Consultancy, Honoraria, Other: Travel, Accomodations, Expenses, Research Funding; Agios: Research Funding; Bayer: Research Funding; Incyte: Research Funding. Gerber:Seattle Genetics: Membership on an entity's Board of Directors or advisory committees; Spectrum: Membership on an entity's Board of Directors or advisory committees; Alexion: Membership on an entity's Board of Directors or advisory committees; Janssen: Research Funding. Grunwald:Medtronic: Equity Ownership; Incyte Corporation: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Alexion: Membership on an entity's Board of Directors or advisory committees; Ariad: Membership on an entity's Board of Directors or advisory committees; Forma Therapeutics: Research Funding; Amgen: Research Funding; Janssen: Research Funding. Kota:Pfizer: Membership on an entity's Board of Directors or advisory committees; Ariad Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Leukemia Lymphoma Society: Research Funding; Incyte: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal