Abstract
Introduction
In their lifetime, patients with follicular lymphoma frequently require multiple treatments, which have improved their survival over the past few decades. The expected treatment outcome based on lines of treatment in the post-Rituximab era is currently unknown. We analyzed the progression free survival and event free survival by line of treatment to aid estimating clinical endpoints when designing future clinical trials for multiply relapsed patients.
Patients and Methods
Adults (≥18 years) with de novo follicular lymphoma (FL) treated at our center between 1998 and 2007 were eligible (N=1134). 236 patients with ≤2 visits, mixed histology at initial diagnosis, and active concurrent malignancy were excluded. Of the remaining 898 patients, 105 were observed and did not require treatment during the timeframe of this dataset, and 2 had incomplete data, therefore 791 patients were eligible for response, progression and event free survival (PFS and EFS) analysis (Figure 1). Response was documented by investigators based on clinical or radiographic assessment. Complete response was based on radiographic assessment. PFS was defined as start of treatment to progression of disease or death. Patients with inadequate response to treatment, change of treatment, or stable disease without subsequent documented relapse were censored in the PFS analysis. Events for EFS were defined as progression, change of treatment, and death. PFS and EFS of sequential lines of treatment were evaluated by Kaplan-Meier method and compared across lines using log-rank test with adjustment for within-patient correlation. PFS and EFS were compared by other clinical variables using regular log-rank tests.
Results
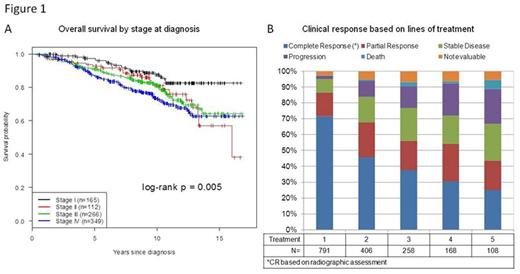
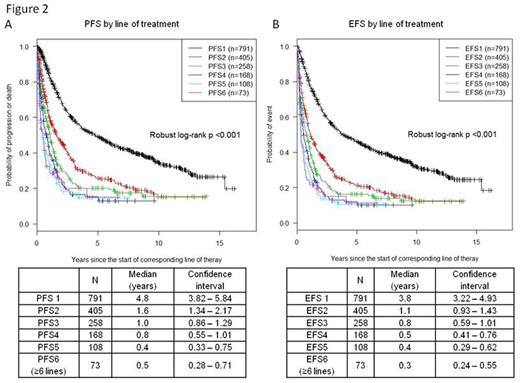
Median age of diagnosis was 57.3 years with 1:1 male to female ratio. Median overall survival was not reached with median follow up of 9 years (N=898, range 0.2 - 16.8 years, Figure 1A). Median time to first treatment for the entire group was 2.3 months (range 0 - 13.3 years). In first line treatment of the 791 patients, 51% (N=406) received Rituximab with chemotherapy (R-Chemo), 13% (N=101) received chemotherapy only (Chemo), 19% (N=150) received Rituximab monotherapy (R-Mono), and 17% (N=129) received other treatments including radiation and surgery. For second line treatment, 405 patients were treated with about 37% receiving R-Chemo and 34% receiving R-Mono. As line of treatment increased, the percentage of patients with radiographically assessed complete response diminished from 71% at first line treatment to 25% by fifth line treatment (Figure 1B). Median PFS for first, second and third line treatment are 4.8, 1.6, and 1 year, respectively (Figure 2A). Median EFS for first, second and third line treatment are 3.8, 1.1, 0.8 year, respectively (Figure 2B). For subsequent lines of treatment, both median PFS and EFS were <1 year.
Conclusion
Follicular lymphoma is an indolent disease often requiring multiple lines of treatment. However, PFS and EFS for multiple lines of treatment in FL has not been described in the post-Rituximab era. The work has benchmarked the median response by line of treatment. After third line treatment, the PFS was ≤1 year. This analysis serves to aide comparison of different therapies for future drug approval in relapsed FL.
Hamlin:Xencor: Membership on an entity's Board of Directors or advisory committees; Portola: Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees; Molecular Templates: Research Funding; Novartis: Research Funding; Seattle Genetics: Research Funding; Gilead: Membership on an entity's Board of Directors or advisory committees. Horwitz:Spectrum: Consultancy, Research Funding; Bristol-Myers Squibb: Consultancy; Huya: Consultancy; Infinity: Consultancy, Research Funding; Kyowa Hakka Kirin: Consultancy, Research Funding; Takeda: Consultancy, Research Funding; Seattle Genetics: Consultancy, Research Funding; Celgene: Consultancy; ADCT Therapeutics: Research Funding. Kumar:Celgene: Honoraria, Other: Scientific Advisory Board; Celgene: Research Funding; Adaptive Biotechnologies: Research Funding; Seattle Genetics: Research Funding; Pharmacyclics: Research Funding. Moskowitz:Merck: Honoraria; Seattle Genetics: Honoraria, Research Funding; Bristol Myers Squibb: Honoraria. Moskowitz:Merck: Membership on an entity's Board of Directors or advisory committees, Research Funding; Bristol-Myers Squibb: Membership on an entity's Board of Directors or advisory committees, Research Funding; Seattle Genetics: Membership on an entity's Board of Directors or advisory committees, Research Funding. Palomba:Pharmacyclics: Consultancy. Zelenetz:Gilead Sciences: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal