Abstract
Background: Ponatinib, an oral tyrosine kinase inhibitor with potent activity against native and mutant BCR-ABL1, is approved for patients with refractory chronic myeloid leukemia (CML) or Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ ALL) for whom no other tyrosine kinase inhibitor (TKI) therapy is indicated, or for patients with the T315I mutation.
The efficacy and safety of ponatinib in patients with resistant/refractory hematologic malignancies were evaluated in a phase 1 trial (NCT00660920). Here, we report 4-year follow-up data from chronic-phase (CP)-CML patients; final data (approximately 5-year follow-up) will be presented.
Methods: In this open-label, dose-escalation, phase 1 trial, 81 patients with resistant/refractory hematologic malignancies (CP-CML, 43 patients; accelerated-phase CML, 9 patients; blast-phase CML, 8 patients; Ph+ ALL, 5 patients) were enrolled. Patients were treated with ponatinib at a starting dose of 2 mg/d - 60 mg/d; intra-patient dose escalation was permitted. In Oct 2013, dose reduction instructions were provided in response to an observed accumulation of arterial occlusive events (AOEs) with longer follow-up across the ponatinib clinical program. For data presented herein, the data cutoff date is 2 Feb 2015, with median follow-up of 53.1 months (range, 1.7 - 69.9 months) for CP-CML patients.
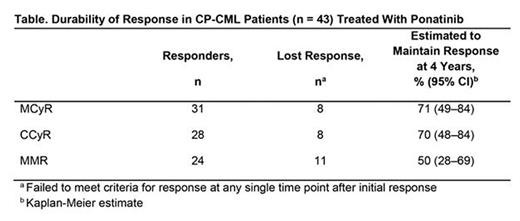
Results: Among CP-CML patients, at baseline, median age was 55 years and median time since diagnosis was 6.6 years; BCR-ABL1 kinase domain mutations were reported in 63% of patients, with T315I confirmed at a central laboratory in 28% of patients. Patients were heavily pretreated, with 37% having received 2 prior TKIs and 60% having received ≥3 prior TKIs. Of 43 CP-CML patients, 22 (51%) remained on ponatinib treatment at data cutoff. Adverse events (AEs; 26%) and disease progression (9%) were the most common reasons for discontinuation of treatment. Cumulative response rates were: major cytogenetic response (MCyR), 72%; complete cytogenetic response (CCyR), 65%; major molecular response (MMR; assessed at a central laboratory), 56%; molecular response 4 (MR4), 42%; MR4.5, 28%. Responses were durable (Table), with median durations of response not reached for MCyR, CCyR, and MMR. Among patients who received ponatinib at starting doses of ≤30 mg/d (n = 15), MCyR was achieved by 67%, CCyR by 53%, and MMR by 47%; ponatinib dose was ≤30 mg/d in all but one of these patients at the time of response. Of 19 patients who were ongoing and in MCyR as of Oct 2013, 13 had their dose reduced; all 13 dose-reduced patients maintained MCyR at data cutoff. Of the 22 ongoing patients at the time of the present analysis, 18 (82%) had CCyR and 17 (77%) had MMR or better (MMR, 6 patients; MR4, 1 patient; MR4.5, 9 patients; MR5, 1 patient) as their response at the data cutoff; 14/22 (64%) ongoing patients were receiving 15 mg/d as their current dose as of the data cutoff. Rash (65%), fatigue (63%), abdominal pain (58%), headache (58%) and arthralgia (53%) were the most common treatment-emergent AEs. The incidence of AOEs (any/serious) was 40%/30% (by subcategory: cardiovascular, 30%/21%; cerebrovascular, 9%/7%; peripheral vascular, 14%/9%).
Conclusions: With median follow-up of over 4 years in this phase 1 study, ponatinib continues to provide clinical benefit to heavily pre-treated CP-CML patients, approximately half of whom continue to receive ponatinib, with a majority in deep response that has been long-lasting; final study data will be presented. The most common treatment-emergent AEs were consistent with the AE profile across the clinical program. Potential for long-term benefit, demonstrated herein, versus risk should be considered when using ponatinib in this patient population.
Study sponsor: ARIAD Pharmaceuticals, Inc.
Mauro:BMS: Consultancy, Honoraria; ARIAD: Consultancy, Honoraria; Novartis: Consultancy, Honoraria, Research Funding; Pfizer: Consultancy, Honoraria. Cortes:ARIAD: Consultancy, Research Funding; Bristol-Myers Squib: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Teva: Research Funding. Kantarjian:Bristol-Myers Squibb: Research Funding; Amgen: Research Funding; ARIAD: Research Funding; Pfizer Inc: Research Funding; Delta-Fly Pharma: Research Funding; Novartis: Research Funding. Shah:ARIAD: Research Funding; BMS: Research Funding; Daiichi-Sankyo: Research Funding; Pfizer: Research Funding; Plexxikon: Research Funding. Flinn:Janssen: Research Funding; Pharmacyclics LLC, an AbbVie Company: Research Funding; Gilead Sciences: Research Funding; ARIAD: Research Funding; RainTree Oncology Services: Equity Ownership. Rivera:ARIAD: Employment, Equity Ownership. Lustgarten:ARIAD: Employment, Equity Ownership. Santillana:ARIAD: Employment, Equity Ownership. Heinrich:Novartis: Consultancy, Patents & Royalties, Research Funding; Pfizer: Consultancy; Bayer: Research Funding; BMS: Research Funding; Blueprint Medicines: Consultancy; MolecularMD: Consultancy, Equity Ownership; ARIAD: Consultancy, Research Funding; Onyx: Consultancy. Druker:Agios: Honoraria; Ambit BioSciences: Consultancy; ARIAD: Patents & Royalties, Research Funding; Array: Patents & Royalties; AstraZeneca: Consultancy; Blueprint Medicines: Consultancy, Equity Ownership, Other: travel, accommodations, expenses ; BMS: Research Funding; CTI: Equity Ownership; Curis: Patents & Royalties; Cylene: Consultancy, Equity Ownership; D3 Oncology Solutions: Consultancy; Gilead Sciences: Consultancy, Other: travel, accommodations, expenses ; Lorus: Consultancy, Equity Ownership; MolecularMD: Consultancy, Equity Ownership, Patents & Royalties; Novartis: Research Funding; Oncotide Pharmaceuticals: Research Funding; Pfizer: Patents & Royalties; Roche: Consultancy. Deininger:Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Gilead: Research Funding; Incyte: Consultancy, Membership on an entity's Board of Directors or advisory committees; BMS: Consultancy, Research Funding; CTI BioPharma Corp.: Membership on an entity's Board of Directors or advisory committees; Celgene: Research Funding; Bristol Myers Squibb: Consultancy, Research Funding; Ariad: Consultancy, Membership on an entity's Board of Directors or advisory committees. Talpaz:Novartis: Research Funding; Incyte Corporation: Other: Travel expense reimbursement, Research Funding; Ariad: Other: Expense reimbursement, travel accomodation expenses, Research Funding; Pfizer: Consultancy, Other: travel accomodation expenses, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal