Abstract
Introduction: Revised prognostic scoring system R-ISS (standard ISS plus cytogenetic changes) has been introduced as a possible tool for evaluation of patients with multiple myeloma. This system is based on pooled data from various clinical trials but has not been validated in patients´ population outside the clinical trial setting.
Aim: To evaluate clinical relevance of R-ISS in real life population of multiple myeloma patients.
Methods: Registry of monoclonal gammopathies (RMG) was established in 2007 and has become one of the flagship projects of the Czech Myeloma Group. The registry collects prospective data from patients with myeloma and other gammopathies (https://trials.cba.muni.cz/trialdb2/interface_forms/login_rmg.asp). Registry is regularly monitored and data are validated by an external monitor. Data from registry were retrieved to identify patients in whom all above mentioned parameters were available. These patients were then stratified according to R-ISS and TTP and OS were calculated as primary endpoints.
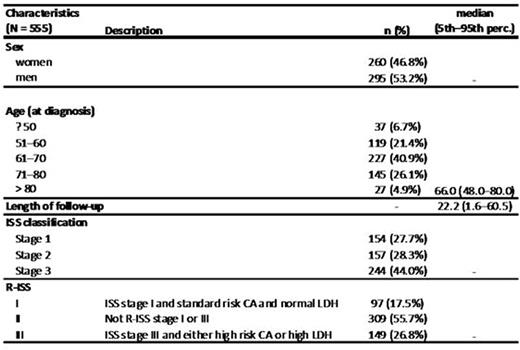
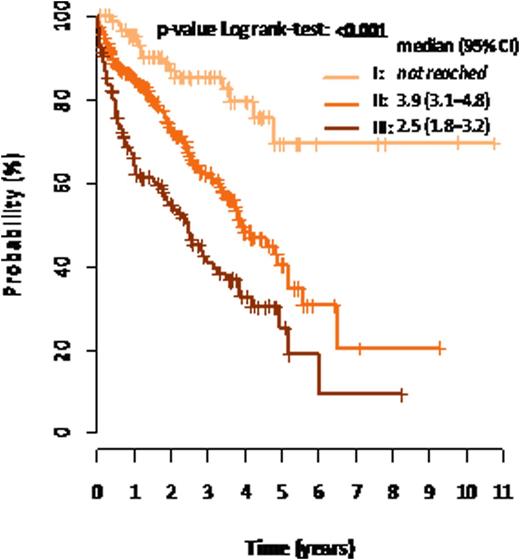
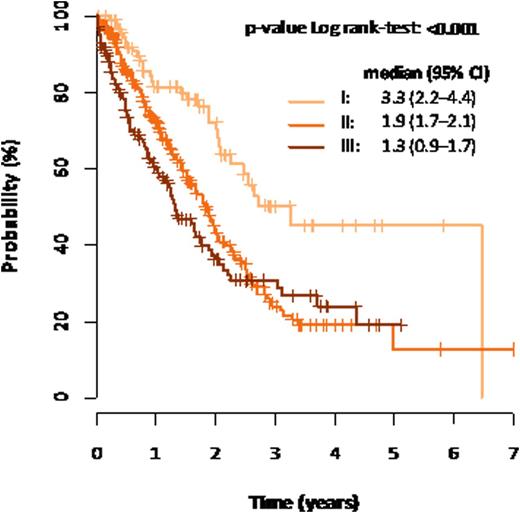
Results: 555 patients (260 females, 295 males, median age 66 years) with multiple myeloma who had full set of necessary data available were identified. Median follow-up of this cohort was 22.2 months. 97 17.5% (97/555) patients were R-ISS stage I, 55.7% 309/555 were R-ISS stage II and 26.8% (149/555) patients were R-ISS III. Median overall survival was not reached for stage I, 3.9 years for stage II and 2.5 years for stage III. The differences were statistically significant (p<0.001, log-rank test). Median time to progression was 3.3 years for stage I, 1.9 years for stage II and 1.3 years for stage III. The differences were statistically significant (p<0.001, log-rank test). Stage I versus II showed HR (95% CI): 2.84 (1.66-4.87), p<0.001 and stage I versus III HR (95% CI): 5.20 (2.99-9.03), p<0.001 for overall survival and HR (95% CI): 2.02 (1.37-2.96), p<0.001 and (95% CI): 2.49 (1.64-3.77), p< 0.001 for time to progression. Similar survival pattern can be seen in a subgroup of patients treated with autologous stem cell transplantation, without autologous stem cell transplantation and the system provides valuable information even in a subgroup of patients who were never treated with novel agents. Figure 1 shows overall survival and figure 2 time to progression of the cohort.
Conclusion: Revised ISS provides valuable information about the long term prognosis in a mixed cohort of real life multiple myeloma patients. This system enables to estimate the prognostic category of each specific patient in a horizon of several years ahead.
Supported by PRVOUK P37.
Spicka:BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen Cilag: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Honoraria; Millenium: Honoraria. Hájek:Janssen: Honoraria; Celgene: Consultancy, Research Funding; BMS: Honoraria; Amgen: Consultancy, Honoraria, Research Funding; Takeda: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal