Abstract
Introduction: Allogenic hematopoietic stem cell transplantation (SCT) is often the only curative treatment for a part of patients with malignant or benign hematological disorders. The availability of a HLA matched related or unrelated donor remains a major obstacle which can be resolved by the presence of alternative donors, like umbilical cord blood, partially matched unrelated or haploidentical family donors. T cell repleted SCT using haploidentical donors (H-SCT) has considerably improved over the last years due to better control of Graft Versus Host Disease (GVHD) using post-transplantation cyclophosphamide (PTCY).
Our study aims to shed light on the interest of chimerism evaluation after H-SCT and to elucidate the cause of graft failure (GF) in our H-SCT recipients.
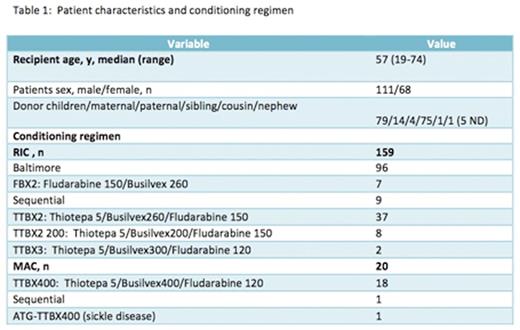
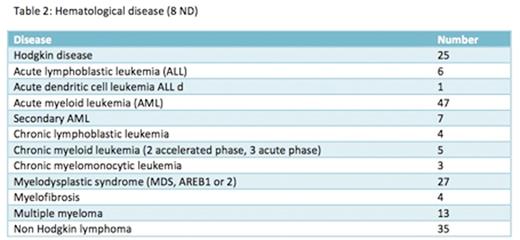
Patients and methods: We conducted a retrospective study of 179 patients (pts) who received a H-SCT in our center from august 2009 to march 2016. The pts characteristics are reported in Table 1 and 2. One hundred sixty pts received a Reduced Intensity (RIC) and 19 pts a MyeloAblative Conditioning (MAC) regimen. Twenty-eight pts had refractory or relapsed acute myeloid leukemia. All pts received PTCY on days 3 and 4 and Ciclosporine A and mycophenolate of mofetil since day 5 for GVHD prophylaxis. Twenty pts received H-SCT for relapse after a first SCT with a HLA identical donor. Stem cell sources were bone marrow (BM) for 19 pts, peripheral blood stem cells (PBSC) for 157 pts and BM+PBSC for 3 pts. The donors were children (79 daughters sons), parents (4 fathers, 14 mothers), family members (75 siblings, 1 cousin, 1 nephew) and 5 unknown.
Chimerism analysis: The peripheral blood CD3 positive cells were selected using the kit Human CD3 Positive selection (Stemcell) and genotyping was performed with the PowerPlex 18D System (Promega), a multiplex STR system allowing the co-amplification of 18 loci.
DSAs (donor-specific anti-HLA antibodies): The DSAs were identified with the use of highly sensitive solid-phase immunoassays. Desensitization therapy with Bortezomib, Rituxan and plasmapheresis was performed in two patients with a high level of DSAs.
Results: Chimerism could be evaluated in 167 patients, where 162 had ≥ 98% of donor cells at a median time of 35 d post-transplant (range 15-170) without secondary GF.
One patient suffering from sickle disease had stable mixed chimerism (82% donor). Only 4 pts had primary GF (2%), all of donor-recipient pairs were ABO compatible, all of them received a Baltimore conditioning whereas 2 pts had BM as SC source. Recipients with PBSC as SC source had poor CD34+ cells infused (1,2 and 2,3 x 106/kg). One of the 4 pts had a high level of DSAs. A patient with mycosis fungoide had secondary GF after relapse (PBSC/DSAs negative).
One patient with a high level of DSAs (78%) had a primary graft failure. Two pts with a high level of DSAs who were desensitized by the described procedure. Forty-five patients had a positive anti-HLA antibody no specific to donor with a median level of 3% (range: 1-45%) with no impact on engraftment (Anti-HLA antibody class I in 30 pts, class II in 11 pts and mixed in 4 pts).
The median number of CD34+ cell count was 5,2x 106/kg (range: 1,3-17) in 160 pts who had PBCS as SC source.
With a median follow up of 532 d (range: 164-1587d), 123 pts are alive and 116 of them are in CR.
Conclusion: Full donor chimerism is obtained in almost all pts after T cell repleted H-SCT with PTCY. Primary graft failure occurred principally in pts with high levels of DSAs and poor graft CD34+ cells. Chimerism analysis post H-SCT is not crucial and might focus on a small group of high-risk pts. Detection of DSAs is crucial in HLA mismatched transplants not only to select the most appropriate donor but also to include a pre-transplantation strategy of desensitization to minimize the risk of graft failure.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal