Abstract
Background:
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is a potentially curative approach for a wide range of hematologic malignancies. However, the period of prolonged immunodeficiency after this procedure results in significant morbidity and mortality from infectious complications. Epstein-Barr virus (EBV) is a latent γ-herpes virus with B lymphocyte-specific tropism that infects more than 90% of healthy individuals. Following allo-HSCT, EBV reactivation and EBV-related proliferations may be associated with a spectrum of clinical presentations, going from fever to lymphoproliferative diseases (LPD), which arise as a consequence of an outgrowth of B cells latently infected with EBV in the setting of loss or suppression of normal cytotoxic T-cell surveillance. Reduction in immunosuppression and/or pre-emptive therapy with rituximab are strategies nowadays employed to prevent or to treat EBV-associated LPD, which have led to a significant improvement in patient outcome. While the role of T cells and NK cells in graft-versus-leukemia (GVL) immune responses has been established, recent studies have shown a potential contribution of B cells on GVL responses. In this context, the impact of the use of monoclonal antibodies targeting B cells lines on disease control has not been evaluated yet. The aim of this study is to evaluate the incidence of EBV reactivation in patients with hematological malignancies after allo-HSCT, the use of rituximab for its treatment and its impact on the transplantation outcomes.
Patients and methods:
We evaluated 359 consecutive patients with hematological malignancies who received allo-HSCT and were followed in our center between January 2008 and June 2015; there were 218 (61%) males and 141 (39%) females with a median age of 48 years (range: 18-70), 182 (51%) had acute myeloid leukemia, 44 (12%) multiple myeloma, 34 (9%) myelodysplastic syndrome, 30 (8%) Non-Hodgkin lymphoma, 7 (2%) chronic lymphocytic leukemia, 21 (6%) myeloproliferative syndrome, 14 (4%) Hodgkin disease, 13 (4%) chronic myeloid leukemia and 14 (4%) aplastic anemia. At transplantation, 227 (63%) patients were in complete response (CR) or chronic phase (CP) and 132 (37%) were in less than CR or CP. For conditioning regimen, 171 (48%) were myeloablative and 188 (52%) were reduced intensity. EBV DNA levels in blood were detected by quantitative real-time polymerase chain reaction (RQ-PCR) after weekly monitoring up to 3 months after allo-HSCT. EBV-DNA was considered positive when the copies exceeded 1000 copies/ml. EBV therapeutic intervention, firstly concerned gradual reduction of the doses of immunosuppressive drugs, and then rituximab infusion (375 mg/m², four injections weekly) was administered when the copies exceeded 10 000 copies/ml.
Results:
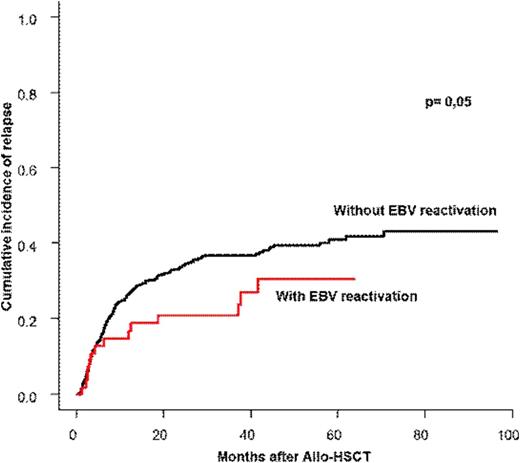
Among 359 patients, 222 patients had EBV reactivation after a median time of 1.3 months (0.7-2.5) after allo-HSCT with a cumulative incidence of 48 % (47-50) at 3 months. Among the 222 patients with EBV reactivation, only 35 (15.7%) needed treatment with rituximab. Rituximab was introduced after a median time of 55 days after transplantation with a median number of 5 infusions and a median dose of 2025 mg/m². EBV treatment was successful in all patients, none progressed to LPD. The cumulative incidence of relapse at one and two years for the whole population was 27% (26-28) and 34% (33-35) respectively and the cumulative incidence of transplant-related mortality (TRM) was 22% (21-23) and 25% (24-26) respectively. The multivariate analysis taking into account the type of disease, the type of conditioning, the use of ATG, the disease status at transplantation, the presence of GVHD and the EBV reactivation with/without rituximab, showed that the presence of EBV reactivation was associated with a significant lower relapse rate, HR= 0.52 [0.35-1], p=0.05. The use of rituximab did not compromise the GVL effect and thus had not impact on relapse or overall survival.
Conclusion:
We showed a positive impact of EBV reactivation on relapse incidence which could be explained as a stimulation of both function and amplification of NK compartment. The strict monitoring of the viral load and therapeutic intervention using rituximab enable a full viral control without any progression into LPD and without any compromise on GVL effect.
Michallet:Bristol-Myers Squibb: Consultancy, Honoraria, Research Funding; Pfizer: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria; Astellas Pharma: Consultancy, Honoraria; MSD: Consultancy, Honoraria; Genzyme: Consultancy, Honoraria. Nicolini:Ariad: Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS: Consultancy, Honoraria; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal