Abstract
Introduction Human Herpes Virus 6 (HHV-6) is an opportunistic and potentially life-threatening infectious agent frequently identified after allogeneic hematopoietic cell transplantation (allo-SCT). In autologous hematopoietic stem cell transplantation (ASCT) setting, although the immune system recovery is rapid and the risk of infection lower in ASCT recipients than allo-SCT ones, cell immunity is impaired and some recipients may develop prolonged myelosuppression, delayed platelet recovery, diarrhea, skin rashes compatible with HHV-6 infection.
The primary objective was to evaluate the incidence of HHV-6 infection in ASCT recipients; secondary objectives were to analyze the clinical relevance of this infection on hematopoietic reconstitution, CMV co-infections and other infectious and non-infectious complications.
Patients and methods
From July 2012 to February 2015, 196 patients undergoing ASCT were enrolled in the prospective multicentric VIRAUTO6 study (NCT02090803). HHV-6 DNA load was measured by quantitative PCR in whole blood specimens once during the 7 days before transplantation and prospectively once a week after transplantation until hematopoietic recovery defined as ANC > 1 x 109/L and platelets > 50 x 109/L with transfusion support cessation. Active HHV-6 and CMV infections were defined as 2 consecutive blood HHV-6/CMV DNAemias ≥ 450 copies/mL, one week apart. Chemotherapy related toxicities were assessed according to the WHO classification.
Results
The median age of this cohort (127 male, 64.8%) was 59.4 (52.5; 64.8) years. Underlying hematological diseases were lymphoma (108, 55.1%), myeloma (86, 43.9%) and leukemia (1, 0.5%). At transplantation, complete remission or very good partial remission were observed in 125 patients (63.8%), and partial response or refractory disease for others (71, 36.2%). Bicnu-Etoposide-Aracytine-Melphalan (BEAM) conditioning regimen was used in 93 patients (47.4%) and melphalan alone conditioning regimen in 87 patients (44.4%). The median follow-up was 16 (14; 20) days. The median ANC and platelets recovery was 9 (7; 12) days and 15 (10; 25) days, respectively.
Twenty-two (11.2%) patients developed active HHV-6 infection with a cumulative incidence of 19.2% at 40 days after transplantation. Among them, only one patient had CMV infection in the same period. The onset of active HHV-6 infection occurred with a median of 13 (12; 15.8) days after transplantation and with a median blood HHV-6 DNAemia of 7,035 (1,192; 19,875) copies/mL. HHV-6 infection occurred less frequently in patients with Melphalan conditioning regimen compared to BEAM alone (OR 0.11; 95%CI 0.02-0.47; p<.001), and was associated to increased non-infectious complications such as mucositis or elevated liver functions tests (OR 5.05; 95%CI 1.78-14.32; p<0.001). Moreover, the severity of these non-infectious complications was higher in recipients exhibiting HHV-6 infection (0R 4.62; 95%CI 1.32-16.2; p=0.006; p<0.01). Nevertheless, there was no statistical difference regarding sex, age, disease status at transplantation and infectious complications (i.e bacterial, fungal, or viral except CMV complications) between the two groups of recipients (with HHV-6 infection vs without).
HHV-6 disease occurred in 3 patients (1.5% of the cohort): 2 patients had skin rash with positive skin biopsy for HHV-6 DNA and one patient had fever with no other cause than HHV-6 infection. For 2 patients, ganciclovir treatment was introduced for a median of 11.5 (9.8; 13.2) days.
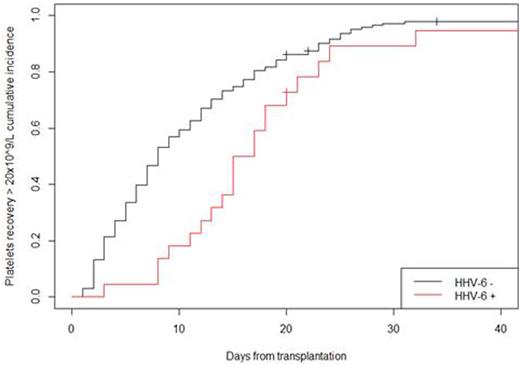
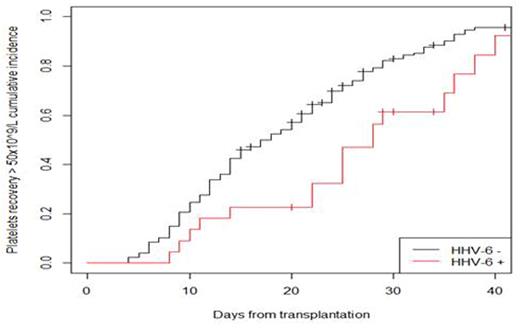
Delayed neutrophils and platelets engraftments were noted with an increased median duration of ANC: 11 (9; 16) vs 9 (7; 12) days; p=0.004) and platelets: 25 (14; 29) vs 15 (10; 24) days; p=0.011 recovery in recipients with HHV-6 infection compared to those without HHV-6 infection. The kinetics of platelets recovery according to HHV-6 infection are shown in figure 1.
Conclusion
This work relates the first prospective multicentric study assessing incidence and clinical impact of HHV-6 infection in ASCT recipients. Our study supports that 11.2% patients undergoing ASCT had HHV-6 Infection with significantly different outcomes, especially delayed hematopoietic reconstitution.
platelets recovery > 20 x 109/L ; p-value = 0.009
platelets recovery > 50 x 109/L ; p-value = 0.007
Augeul-Meunier:janssen: Consultancy; gilead: Consultancy. Salles:Novartis: Consultancy, Honoraria; Gilead: Honoraria, Research Funding; Janssen: Consultancy, Honoraria; Celgene: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Mundipharma: Honoraria; Roche/Genentech: Consultancy, Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal