Abstract
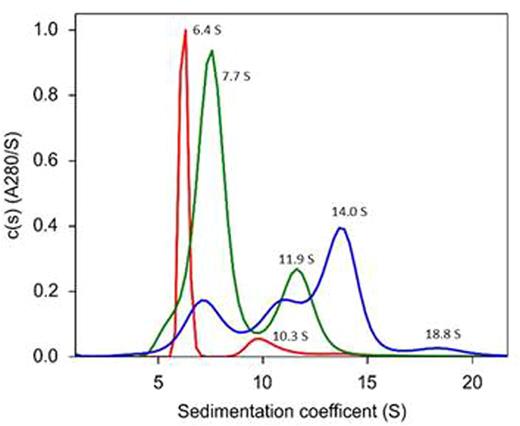
Inhibitory polyclonal IgG antibodies (inhibitors) to factor VIII (fVIII) represent the most significant complication in patients with congenital hemophilia A. FVIII also is the most frequently targeted coagulation factor in autoimmunity. Antibodies recognizing epitopes in the fVIII A2 and C2 domains are present in most inhibitor patients. In the current study, we characterized the hydrodynamic properties of fVIII immune complexes formed by murine anti-human anti-A2 and anti-C2 fVIII monoclonal antibodies (MAbs) 4A4 and 3D12. 4A4 is representative of the most frequently identified group of anti-A2 MAbs identified in the murine hemophilia A immune response to human fVIII. 3D12 is a classical anti-C2 MAb that inhibits the binding of fVIII to von Willebrand factor (VWF) and phospholipid membranes. Velocity sedimentation of immune complexes formed by varying ratios of 4A4 and 3D12 with a high-expression fVIII construct designated ET3 was conducted at 55,000g and 20 °C by measuring protein absorbance at 280 nm in a Beckman XL-I analytical ultracentrifuge. Sedimentation coefficient (s20,w) distributions of fVIII, MAbs and immune complexes were determined using SEDFIT. The sedimentation coefficients of fVIII in the absence of MAbs and of the MAbs in the absence of fVIII were 7.7 S and 6.4 S, respectively. Under conditions of excess MAb (equimolar 4A4 and 3D12 each in five-fold molar excess over fVIII), a 10.3 S immune complex was observed, representing singly-ligated MAbs (Figure, red trace). Under conditions of excess fVIII (fVIII in four-fold molar excess over equimolar 4A4 and 3D12), 11.9 S doubly-ligated MAb complexes were observed (Figure, green trace). A mixture containing equimolar fVIII and 4A4/3D12 MAb binding sites produced a dominant 14.0 S species and a minor 18.8 S species, indicative of cross-linked 3D12-fVIII-4A4 immune complexes (Figure, blue trace). Indefinite association or immunoprecipitation was not observed. These results demonstrate that a biclonal, bivalent anti-fVIII antibody population can form higher-order immune complexes. These complexes may be a driving factor in the immune response to fVIII by promoting B cell activation and/or antigen presentation. Additionally, these results indicate that analytical ultracentrifugation is a useful tool to characterize fVIII immune complexes.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal