Abstract
Background:
Adoptive infusion of T lymphocytes modified with anti-CD19 chimeric antigen receptor (CAR) by viral vectors is a therapeutic option proven effective in the treatment of hematological malignancies. In the context of B-lineage neoplasms, CD19-specific CART cells demonstrated unexpected positive results, achieving complete remission and durable response. To overcome high manufacturing costs, regulatory hurdles and scale-up complexities, we recently established a platform for non-viral gene manipulation of Cytokine-Induced Killer (CIK) cells, an effector T cell population characterized by enrichment in highly cytotoxic CD3+CD56+ cells and reduced risk of GvHD, in compliance with Good manufacturing practices (GMP). Therefore, we investigate whether donor-derived CD19CAR redirected CIK cells can significantly increase anti-leukemic responses in Acute Lymphoblastic Leukemia (ALL) by performing a pre-clinical validation of the non-viral platform.
Methods:
Large-scale production will be performed by nucleofection in presence of two GMP-grade Sleeping Beauty transposon DNA plasmids, coding for the anti-CD19CD28OX40 CAR transgene and for the SB11 transposase. T cells were differentiated according to a procedure derived from the Eudract n 2008-003185-26 study with the addition of a single stimulation with gamma-irradiated PBMCs to rescue the impaired T-cell expansion induced by electroporation.
Results:
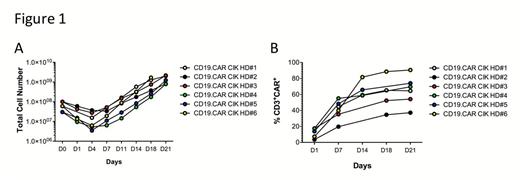
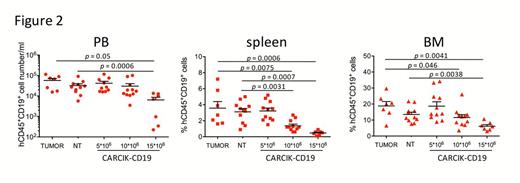
The feasibility of large scale manufacturing process was verified starting from 30-60X10^6 PBMC, reaching efficient expansion with an average of 69.3±15.2-fold increase (Figure 1A) and stable expression of CD19CAR (average 65%, Figure 1B). Immunophenotypic analysis showed the typical enrichment of the CD3+/CD56+ cell subset, and maintenance of naive and central memory CD8+ and CD4+ T cells. Modified cells displayed a specific and effective cytotoxicity and proliferation towards CD19+ cell lines and primary tumors. In order to determine the biological effect of CARCIK-CD19 cells on disease and the correct dose for optimal activity, we performed a CARCIK-cell dose titration in a xenograft mouse model of common BCP-ALL, bearing the feature of a Ph-like gene rearrangement (PAX5/AUTS2). Mice were treated with 5x106, 10x106, 15x106 CAR+ cells or with 10x106 not transduced (NT) cells. CARCIK-CD19 cells showed a dose-dependent antitumor response and persisted in tumor-bearing animals, in peripheral blood (PB), bone marrow (BM) and spleen (Figure 2). Furthermore, to evaluate whether cryopreservation affects the activity of the cell product, we performed functional experiments with both fresh and frozen/thawed CAR CIK cells in vitro and in an established MLL/ENL patient-derived xenograft model. CARCIK.CD19 cells remained functional after freezing and thawing as evidenced by killing activity and decreased level of leukemic engraftment in the spleen of treated mice. Finally, we evaluated CARCIK-CD19 cell bio-distribution, safety and acute toxicity in NOD-SCID-gamma chain-/- (NSG) mice. The infusion of CARCIK-CD19 cells proved to be safe and well tolerated in all mice tested. The infused cells persisted in time in the hematopoietic and post-injection perfused organs with a follow-up of 3 months.
Conclusions:
Our findings describe a novel donor-derived non-viral CAR approach characterized by efficient cell transfection, expansion and functionality that could be used as valid and sustainable alternative to patient-derived viral approach with the aim to increase the cure rate of children and adults with leukemia. This study provides the proof-of-concept for designing phase I/II study for relapsing and refractory ALL post Hematopoietic Stem Cell Transplantation.
Cooper:Ziopharm Oncology: Employment, Equity Ownership, Patents & Royalties; Intrexon: Equity Ownership; City of Hope: Patents & Royalties; Targazyme, Inc.,: Equity Ownership; Immatics: Equity Ownership; Sangamo BioSciences: Patents & Royalties; MD Anderson Cancer Center: Employment; Miltenyi Biotec: Honoraria. Biondi:Cellgene: Other: Advisory Board; Novartis: Membership on an entity's Board of Directors or advisory committees, Other: Advisory Board; BMS: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal