Abstract
Non-specific amplification of background DNA may cause false positive MRD results using conventional Ig/TCR gene rearrangement RQ-PCR; non-amplification control (NAC) samples taken from pooled buffy coat lymphocytes are thus routinely analysed in parallel to test samples. In contrast NGS-based MRD relies solely on DNA sequence specificity to avoid false positives. However at certain alleles gene rearrangement diversity is limited and false positive results remain a risk (the same DNA sequence from normal not leukaemic lymphocytes). We therefore assessed the relevant parameters of Ig/TCR gene rearrangement (5'/3' gene selection, deletion/insertion size) across all loci screened by the Biomed-2 PCR panel, in pooled buffy coat (24 donors), single blood donor cones and patient samples at end of induction (EOI). This subsequently allows us to determine an 'e-score', the probability that a given sequence is generated by chance. This allows investigators to avoid selecting gene rearrangements with poor diversity for MRD analysis in a systematic manner.
In a 1st round PCR DNA was amplified with primers covering 6 Ig/TCR loci (IgH, inc. IgH, IgK, TRB, TRD and TRG) containing partial MiSeq adaptor sequences. The resulting amplicons were purified using Agencourt AMPure XP beads (Beckman Coulter, Jersey City, NJ). The amplicons acted as templates in a 2nd round PCR, using primers containing index and full MiSeq adaptor sequences. Samples were purified, then quantitated by Qubit (InvitroGen, Carlsbad, CA) and TapeStation analysis (Agilent, Santa Clara, CA). This data was used to normalise DNA and construct the final sequencing library which was quantified using the Kapa Library Quantification Kit (Kapa Biosystems, Wilmington, MA) to ensure ideal cluster density. PhiX Sequencing Control v3 (Illumina, San Diego, CA) was added to the sequencing library at 8% to ensure early cycle high sequence diversity. Bi-directional sequencing was completed using Illumina MiSeq 500 cycle cartridge and Nano Reagent Kit (Illumina, San Diego, CA). The Vidjil bioinformatic pipeline (Bonsai team, CRIStAL, Lille, France) was used for data analysis.
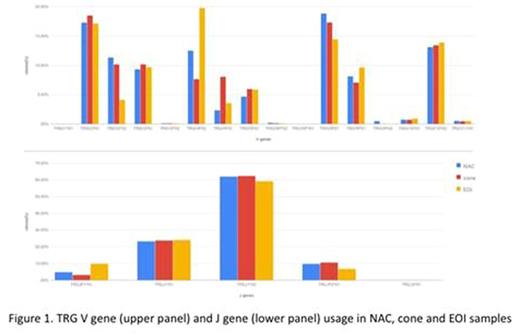
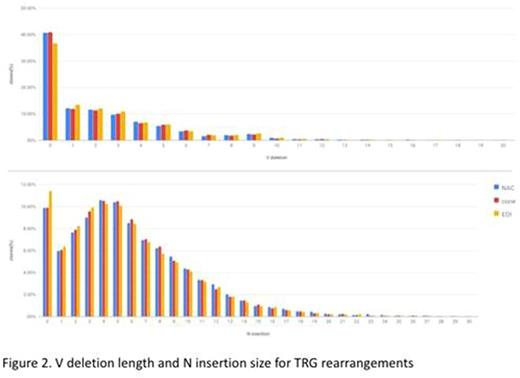
As illustrated in Fig 1 for TRG, 5' and 3' allele selection was found to be biased at all loci. However, the biases were stable across NAC, cone and importantly EOI samples. EOI samples were somewhat more oligoclonal than those of normal individuals (146 vs 212 vs 255 clones/1000 reads for EOI, NAC and cone respectively) leading to slightly greater variance around mean allele usage. Mean 5' and 3' deletion lengths and N insertion size varied both across loci and between alleles of a given gene. The patterns were stable across NAC, cone and EOI samples (fig 2).
As the data from NAC samples is representative of background DNA in typical end of induction test samples, an e-score for defining rearrangement probability is therefore calculable (fig 3).
Example e-scores for typical rearrangements are: -
VH2.5-DH3.22-JH4.02 (-4/7/-7)(-5/9/-6) = 2.03x10-11
DD2-DD3 (-3/5/-3) = 1.06x10-4
TRBV6-2*01-TRBD2*01-TRBJ2-3*01 (-3/7/-4)(-2/10/-5) = 2.05x10-11
VKINT-KDE (-5/6/-1) = 9.94x10-5
IGKV3-20*01 (-3/5/-6) KDE = 4.32x10-6
The worst e-score would be found for rearrangements with poor diversity including IGK-KDE rearrangements (VKInt-KDE 0/0/0 e = 1.7x10-01) and certain TRG rearrangements (V8*01/J1*02 0/0/0 e=1.3x10-03). In contrast the worst e-score for IgH (V3-23*01-D6-13-J4*02(0/0/0)(0/0/-4) is 3.5x10-07.
Incorporating a 1 log margin of safety discrimination of specific amplification over background amplification as per Ig/TCR RQ-PCR MRD criteria would mean that an e-score of <1x10-07 is required to confirm a true negative result at 1 in 1,000,000 cells. Thus in practice the vast majority of IgH rearrangements meet those criteria, whilst only a minority of IGK and TRG rearrangements (<10%) would achieve an e-score of 10-05, low enough to be used as a single target for conventional MRD stratification at the commonly used threshold for low risk disease of 10-04). Nevertheless, a multi-loci approach for MRD target identification incorporating loci likely to yield poorer e-scores remains a worthwhile strategy that increases the probability of finding an MRD target in a given patient.
In conclusion the e-score provides a standardised methodology for robust target selection that is useful for quality control purposes and one that should be incorporated into clinical NGS-MRD pipelines.
Moppett:Jazz Pharmaceuticals: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal