Abstract
While there have been extensive studies to define the roles of recurrent somatic mutations in AML, the contribution of germline variants to AML initiation and progression is less well established. DNA repair disorders often predispose patients to developing myeloid malignancies. In particular, biallelic mutations affecting FANC genes cause the recessive heritable bone marrow failure syndrome Fanconi Anemia (FA), which is associated with >800-fold increased risk of progression to AML. A recent explosion of cancer predisposition studies has also revealed the importance of germlineFANC variants in elevated cancer risk (Cancer Treat Rev 2012; 38:89). To investigate the role of FANC gene variants in AML we have performed a case-control study, analyzing rare, deleterious somatic and germline variants for the 19 FANC genes in adult AML and healthy controls cohorts.
Whole exome sequencing was performed on diagnosis samples from 131 adult Caucasian AML patients from two major Australian centers, and a cohort of 329 healthy females. We identified rare Tier 1 variants using a minor allele frequency (MAF) < 0.001, as reported in common dbSNP137, 1000 Genome and NHLBI-ESP project databases. Combined Annotation Dependent Depletion algorithm (CADD, Nat Genet 2014; 46: 310) >10 was used to filter for FANC gene variants with high probability of pathogenicity. Sanger sequencing of matched tumour/non-tumour DNA showed the large majority of variants tested to be germline (90%), consistent with previous studies reporting that somatic FANC genes variants are extremely rare in AML (< 1%). Overall, we identified 52 FANC gene variants in 44 cases with 34% of AML cases carrying one or more variant.
For independent validation we determined the presence of somatic and germline FANC variants in the TCGA AML cohort using an identical pipeline and filtering analysis. In line with our results, we found that 36% of TCGA AML patients carry at least one germline FANC variant. We investigated known disease-causing (D-C) variants in these two AML cohorts using the FA (FAMutdb) and breast cancer (kConFab and BIC) mutation databases. We found 8 D-C FANC variants in the Australian AML cohort and 5 in the TCGA cohort, with 1 variant present in both cohorts. Moreover, the frequency of D-C variants in our cohort of females with AML (n=51) is 13.7%, while the frequency in the healthy female cohort is 4.5%, comparable to that reported in the ESP database for female European-Americans (2.1%, Hum Mol Genet 2014; 23: 6815). Accordingly, we determined that deleterious FANC germline variants confer a significant increased risk of AML (P=0.018, OR=3.3 for the Australian AML cohort).
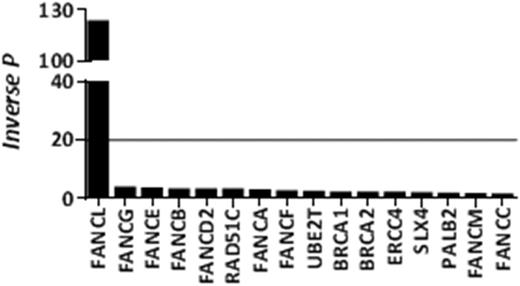
Finally, we performed mutational burden analysis to investigate enrichment of variants associated with particular FANC genes across the AML cohort. This revealed a significant enrichment of FANCL variants in AML vs healthy controls (P=0.008, Figure 1). FANCL is the enzymatic component of the FA core complex that monoubiquitinates the FANCD2/I heterodimer initiating DNA repair, and its down-regulation has been linked to AML (Oncogene 2016; doi:10.1038). Several FANCL variants, found in our AML cohort, affect the catalytic RING domain and are of particular interest. These include a D-C null variant present in 2 patients, a frame shift variant in 2 patients who presented with AML at a very early age (27 and 46 years old), and a variant affecting a critical conserved residue required for monoubiquitination of FANCD2/I.
In conclusion, we show enrichment of rare potentially deleterious FANC gene mutations in AML, associated with a 3-fold increased risk of developing the disease. We hypothesise that, in hematopoietic stem/progenitor cells, these variants confer a subtle defect in interstrand cross-link repair leading to an increased accumulation of mutations and subsequent development of AML. Consistent with this there have been several reports of defective DNA damage repair and increased sensitivity to DNA damaging agents in cells from FANC carriers compared to normal controls (Nat Commun 2014; 5:5496; Mutagenesis 2009; 24:67). Importantly, it is possible to target defects in several DNA repair pathways, and our finding identifies a group of AML patients who may benefit from approaches that target defective FA and homologous recombination pathways.
A significant increase mutational burden of FANCL was observed in our AML cohort (line represents P=0.05).
A significant increase mutational burden of FANCL was observed in our AML cohort (line represents P=0.05).
Gill:Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal