Abstract
Background: Bone marrow (BM) fibrosis is a major cause of morbidity and mortality in patients with myeloproliferative neoplasms. Its progression correlates with thickening and distortion of the bone trabeculae, a phenomenon commonly referred to as osteosclerosis. The physiological process of bone remodeling depends on a delicate interplay between monocyte-derived bone-resorbing osteoclasts and mesenchymal cell-derived bone-forming osteoblasts. Whereas impairment in bone resorption is believed to be the underlying cause of inherited sclerosing bone dysplasias, little is known about the pathogenesis of osteosclerosis associated with myelofibrosis (MF). Phenotypically complex bone-remodeling cells are attached to the bone surface and as such are difficult to study by conventional techniques. Here, we present a novel method for visualization and quantification of these cells in situ and assess their role in inducing osteosclerotic changes in the BM of patients with MF.
Methods: Formalin-fixed paraffin-embedded BM biopsy specimens were obtained from the iliac crest of patients with advanced stages of MF (MF-2 and MF-3) and healthy controls. Staining of each sample was performed using a series of previously validated monoclonal antibodies directed against osteoclast- (TRAP and cathepsin K) and osteoblast-specific antigens (CD56 and RUNX2). To achieve non-interfering identification, we employed a tyramide-based fluorescent immunohistochemistry approach (PerkinElmer Opal). Specimens were imaged in toto using an automated multispectral system at 0.5 µm resolution and segmented to individual cells via a pattern recognition algorithm. Cells were assigned to one of the two phenotypes based on specific signal intensities and size. Finally, cells lying within 20 µm of the bone perimeter were quantified utilizing methods of spatial statistics.
Results: Samples from 10 MF patients (age 63 ± 8 years, median ± MAD) were compared to 3 healthy controls (age 48 ± 7 years). Area of examined tissue ranged from 8.2 to 38.4 mm2 with the median BM to bone ratio of 1.6:1 and 3.5:1, in MF and controls respectively. Discrimination of bone-remodeling subsets by the assay demonstrated high sensitivity and specificity, estimated both visually (Figure 1) and quantitatively. In MF patients we detected 7.1 ± 3.1 osteoclasts per 100 mm of bone perimeter (mean ± SEM) versus 2.4 ± 1.9 per 100 mm in healthy controls (p < 0.0001). Frequency of observable osteoblasts per bone perimeter also showed a significant increase in MF compared to healthy controls (p < 0.0001). Quantification using area of the bone as denominator was in concordance with the densities found per bone perimeter and similar statistical significance was observed.
Conclusions: Our analytical method offers a feasible approach for in situ investigation of complex cellular subsets in the BM. This analysis demonstrates that both osteoclasts and osteoblasts are pathologically affected in advanced stages of MF. Expansion of osteoblastic population supports the involvement of cells of mesenchymal lineage in the progression of osteosclerosis. By contrast, an overwhelmingly increased density of osteoclasts suggests a functional loss of these cells of monocytic origin. This is particularly intriguing in view of the emerging role neoplastic monocyte-derived fibrocytes play in the pathogenesis of MF. Further studies exploiting the interaction between these cells will likely shed light on the pathogenesis of osteosclerosis in MF.
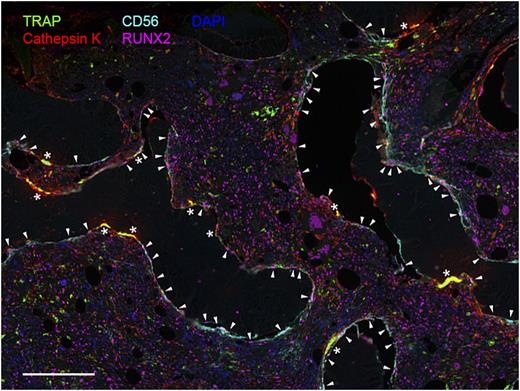
Visualization of bone-remodeling cells in BM tissue section of a patient with MF. Immunostaining with TRAP and cathepsin K revealsgiant multinucleated osteoclasts (*), while elongated CD56+/RUNX2+ osteoblasts (▾) line along the bone edge. Scale bar: 200 µm.
Visualization of bone-remodeling cells in BM tissue section of a patient with MF. Immunostaining with TRAP and cathepsin K revealsgiant multinucleated osteoclasts (*), while elongated CD56+/RUNX2+ osteoblasts (▾) line along the bone edge. Scale bar: 200 µm.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal