Abstract
Introduction: Myelodysplastic syndrome (MDS) is a heterogeneous group of bone marrow disorders with a yearly incidence of approximately 13,000 in the United States. It has been observed that both genetic mutations within stem and progenitor cells and a disordered immune microenvironment are present early in MDS. Abnormal levels of inflammatory cytokines as well increased numbers of suppressive cell types, such as regulatory T cells and myeloid derived suppressor cells (MDSC) have been noted in MDS bone marrow. MDSC are recently discovered subset of myeloid cells with specific immune regulatory functions, such as T cells suppression, seen in pathological conditions, such as cancer. Recent data suggest MDSC may play a critical role in MDS pathogenesis, and that S100A9, a danger-associated molecular pattern (DAMP) produced by some myeloid cells, including neutrophils, monocytes and MDSC, is a key signal for bone marrow immune dysregulation. Here, we report a systems immunology approach to cell type discovery within MDS bone marrow using high dimensional mass cytometry.
Methods: Bone marrow aspirate samples with informed consent from MDS (n=19) and AML (n=4) patients were collected and cryopreserved following red blood cell lysis for storage by the Vanderbilt Hematology Tissue Repository, a tissue repository approved by the local Institutional Review Board (IRB). Samples were acquired for the study and stained with a 35-marker panel of metal tagged mass cytometry antibodies and analyzed with a mass cytometer (CyTOF). Cellular populations were then characterized using biaxial gating as well as viSNE, SPADE and hierarchical clustering as has been previously reported (Diggins et al. Methods 2015, Ferrell et al. PLoS One, 2016).
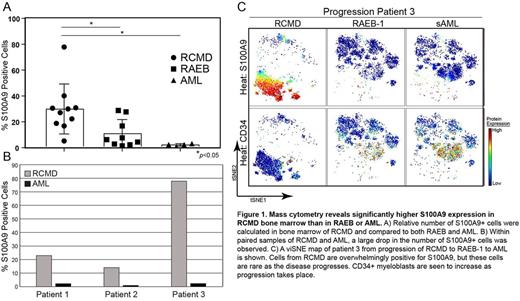
Results: Unsupervised viSNE analysis of 35-markers per cell revealed distinct cellular subsets within each sample. Interestingly, one of the strongest marker signals was expression of S100A9, which was seen in multiple cells types including phenotypic MDSC. Further analysis revealed that as a percentage of bone marrow cells, S100A9 expression was significantly more common in RCMD vs. RAEB and AML (30.0% (n=10) vs. 10.9% (n=9) and 2.4% (n=4), respectively, p<0.05 for each comparison) (Figure 1A). Additionally, three paired RCMD/AML samples were available for analysis. Within these patients, the percentage of S100A9+ cells dropped from a mean of 41.7% in RCMD to a mean of 1.84% in AML bone marrow (Figure 1B&C).
Conclusion: S100A9 is both a distinguishing feature of RCMD and of disease progression within MDS. Because of its important role inflammation and cellular recruitment, S100A9 may correlate with bone marrow cellular inflammation and could represent a viable target in treatment of the disordered immune microenvironment present in MDS, especially RCMD.
Savona:Celgene: Membership on an entity's Board of Directors or advisory committees; Sunesis: Research Funding; Incyte: Membership on an entity's Board of Directors or advisory committees, Research Funding; Ariad: Membership on an entity's Board of Directors or advisory committees; Amgen Inc.: Membership on an entity's Board of Directors or advisory committees; TG Therapeutics: Research Funding; Takeda: Research Funding; Gilead: Membership on an entity's Board of Directors or advisory committees. Irish:Incyte: Research Funding; Janssen: Research Funding; Cytobank, Inc.: Equity Ownership, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal