Abstract
Introduction: The anti-apoptotic protein BCL2 is overexpressed in a number of hematological malignancies, including chronic lymphocytic leukemia (CLL). Venetoclax (VEN) is a selective, oral BCL2 inhibitor with demonstrated activity and an acceptable safety profile both as monotherapy and in combination regimens in relapsed/refractory (R/R) CLL patients (pts). VEN has demonstrated preclinical activity with bendamustine/rituximab (BR), a standard treatment in this population, as well as clinical efficacy with R. The anti-CD20 mAb obinutuzumab (G) has demonstrated improved efficacy in combination with chlorambucil compared with R. Here we present an interim analysis of an ongoing phase 1b study (GO28440) evaluating the maximum tolerated dose (MTD) of both VEN+BR and VEN+BG, plus safety, tolerability, efficacy and optimal order of administration in both R/R or 1L CLL pts (NCT01671904).
Methods: Pts with ECOG PS ≤1, 0-3 prior chemotherapy lines, and adequate marrow, hepatic, renal and coagulation function are being enrolled in dose finding cohorts (VEN 100-400mg/day +BR or +BG in a 3+3 dose escalation design) and subsequent safety expansion cohorts (400mg). Dose finding occurs in one of two dosing schedules: Schedule A (VEN introduced before BR or BG) or Schedule B (BR or BG introduced before VEN), with one chosen for expansion per unique population/drug grouping. Both schedules include a gradual VEN dose ramp-up and other prophylactic measures based on risk stratification to reduce the risk of tumor lysis syndrome (TLS). Following completion of 6 months of combination therapy, pts continue single-agent VEN until unacceptable toxicity, disease progression, or (for 1L pts only) up to 1 year total VEN. Dose-limiting toxicity (DLT) data are reviewed after all pts in a cohort have completed 21 days of combination treatment at the target VEN dose and focus on TLS and cytopenias. Efficacy is assessed by iwCLL guidelines; Hallek et al. 2008.
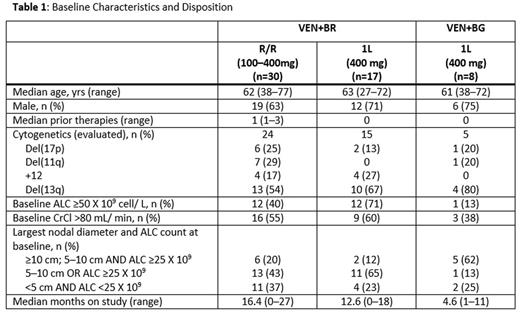
Results: At data cutoff (May 2, 2016), 55 pts (baseline characteristics and disposition in Table 1) have been treated; 47 pts received VEN+BR: 30 R/R (12 on Schedule A at doses 100-400mg, 18 on Schedule B all at 400mg) and 17 1L (all at 400mg: 6 on Schedule A, 11 on Schedule B). Eight pts received VEN+BG: all on Schedule B at 400mg.
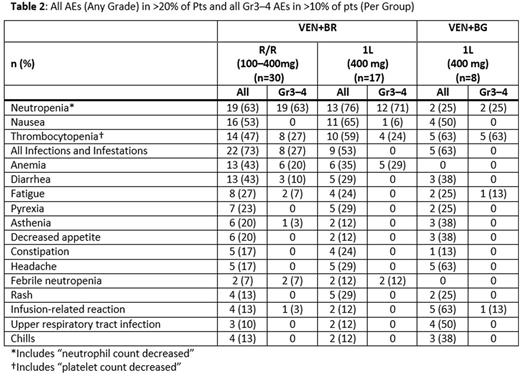
Safety is summarized for VEN+BR (R/R and 1L) and VEN+BG (1L) in Table 2. The most common AEs across all groups were neutropenia, thrombocytopenia, and nausea. Most G3-4 AEs across all groups were hematological toxicities; common non-hematological AEs included diarrhea and fatigue. Platelet transfusions occurred in 4 (13%) R/R VEN+BR and 1 (13%) 1L VEN+BG pts. There were serious AEs in 21 pts (43% R/R VEN+BR, 35% 1L VEN+BR, 25% 1L VEN+BG) including febrile neutropenia, nausea, vomiting, erythema, and infection. No deaths were reported. No TLS events (laboratory or clinical) were observed.
Dose interruptions or modifications for VEN and/or BR/BG occurred in 35 (75%) of VEN+BR pts (22 R/R, 13 1L) and 7 (88%) VEN+BG pts, mainly due to neutropenia. Early VEN discontinuations for toxicity: 7 pts VEN+BR (R/R), 2 pts VEN+BR (1L), 1 pt VEN+BG. Early B and/or R/G discontinuations for toxicity: 10 pts VEN+BR (R/R), 4 pts VEN+BR (1L), 2 pts VEN+BG. Common reasons for discontinuations (≥1 pt) included neutropenia and thrombocytopenia. Across all pts, a median of 4 cycles of B were completed.
Best overall response and minimal residual disease (MRD) status for evaluated pts are presented in Table 3. All but one R/R pt (off study early for disease transformation) responded across all groups.
Conclusion: This study is the first to evaluate both VEN+BR and VEN+BG in pts with CLL. VEN 400mg daily can be combined with BR or BG in patients with either R/R or 1L CLL; neutropenia and thrombocytopenia are manageable with an otherwise acceptable safety profile. Administration schedules (VEN or BR first) appear to have similar toxicity profiles, with no DLT or lab/clinical TLS reported for either during dose finding. As expected, due to the known safety profile of VEN and B, hematologic toxicity was observed with VEN+BR; early VEN+BG safety data appears similar. Events appeared manageable, although early discontinuations contributed to a median of 4 completed cycles of B across all pts. Even with median of <6 cycles of BR, all evaluated pts responded, with more than half showing CR and/or undetectable MRD. Responses may continue to deepen as more data is collected; the study is still enrolling.
Stilgenbauer:Pharmacyclics: Consultancy, Honoraria, Other: Travel grants , Research Funding; Genentech: Consultancy, Honoraria, Other: Travel grants , Research Funding; Sanofi: Consultancy, Honoraria, Other: Travel grants , Research Funding; AbbVie: Consultancy, Honoraria, Other: Travel grants, Research Funding; Amgen: Consultancy, Honoraria, Other: Travel grants, Research Funding; Boehringer Ingelheim: Consultancy, Honoraria, Other: Travel grants , Research Funding; Celgene: Consultancy, Honoraria, Other: Travel grants , Research Funding; Mundipharma: Consultancy, Honoraria, Other: Travel grants , Research Funding; GSK: Consultancy, Honoraria, Other: Travel grants , Research Funding; Hoffmann-La Roche: Consultancy, Honoraria, Other: Travel grants , Research Funding; Genzyme: Consultancy, Honoraria, Other: Travel grants , Research Funding; Janssen: Consultancy, Honoraria, Other: Travel grants , Research Funding; Novartis: Consultancy, Honoraria, Other: Travel grants , Research Funding; Gilead: Consultancy, Honoraria, Other: Travel grants , Research Funding. Morschhauser:Gilead Sciences: Consultancy, Honoraria; Roche: Consultancy, Honoraria; Celgene: Consultancy, Honoraria; Janssen: Honoraria; Servier: Consultancy, Honoraria. Wendtner:Novartis: Consultancy, Honoraria, Research Funding; Genentech: Consultancy, Honoraria, Research Funding; Hoffmann-La Roche: Consultancy, Honoraria, Research Funding; Gilead: Consultancy, Honoraria, Research Funding; Servier: Consultancy, Honoraria, Research Funding; AbbVie: Consultancy, Honoraria, Research Funding; Glaxo-SmithKline: Consultancy, Honoraria, Research Funding; Janssen-Cilag: Consultancy, Honoraria, Research Funding; Mundipharma: Consultancy, Honoraria, Research Funding. Cartron:Roche: Consultancy, Honoraria; Celgene: Honoraria; Gilead: Honoraria; Jansen: Honoraria. Hallek:Gilead: Consultancy, Honoraria, Other: travel support, Research Funding, Speakers Bureau; AbbVie: Consultancy, Honoraria, Other: travel support, Research Funding, Speakers Bureau; Celgene: Consultancy, Honoraria, Other: travel support, Research Funding, Speakers Bureau; Amgen: Consultancy, Honoraria, Other: travel support, Research Funding, Speakers Bureau; Janssen-Cilag: Consultancy, Honoraria, Other: travel support, Research Funding, Speakers Bureau; F. Hoffmann-LaRoche: Consultancy, Honoraria, Other: travel support, Research Funding, Speakers Bureau; Mundipharma: Consultancy, Honoraria, Other: travel support, Research Funding, Speakers Bureau. Eichhorst:Gilead: Consultancy, Honoraria, Other: travel support, Research Funding, Speakers Bureau; GlaxoSmithKline: Consultancy, Honoraria, Other: travel support, Research Funding, Speakers Bureau; Mundipharma: Consultancy, Research Funding, Speakers Bureau; Roche: Consultancy, Research Funding, Speakers Bureau; Celgene: Consultancy, Honoraria, Other: travel support, Research Funding; AbbVie: Consultancy; Janssen-Cilag: Consultancy, Honoraria, Other: travel support, Research Funding, Speakers Bureau. Kozloff:AbbVie: Consultancy, Speakers Bureau; Genentech: Consultancy, Speakers Bureau; Celgene: Consultancy, Speakers Bureau. Lozanski:Genentech: Research Funding; Boehringer Ingelheim: Research Funding; Beckman Coulter: Research Funding; Stemline Therapeutics Inc.: Research Funding. Punnoose:Genetech, Inc.: Employment. Wang:Genetech, Inc.: Employment. Hilger:Genetech, Inc.: Employment. Mobasher:Genentech, Inc.: Employment. Salles:Celgene: Consultancy, Honoraria; Janssen: Honoraria; Gilead: Consultancy, Honoraria; Roche: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria; Amgen: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal