Abstract
Solitary bone plasmacytoma (SBP) is characterized by a solitary area of lytic bone destruction due to clonal plasma cell proliferation without evidence of systemic disease spread. The greatest risk is the progression to multiple myeloma (MM). The choice of the initial treatment may influence disease evolution. Radiotherapy remains the treatment of choice for SBP, although its efficacy has been tested only in small and historical retrospective series. This retrospective study aimed to evaluate the impact of the adjuvant chemotherapy associated to radiotherapy (combined treatment) on the evolution of SBP.
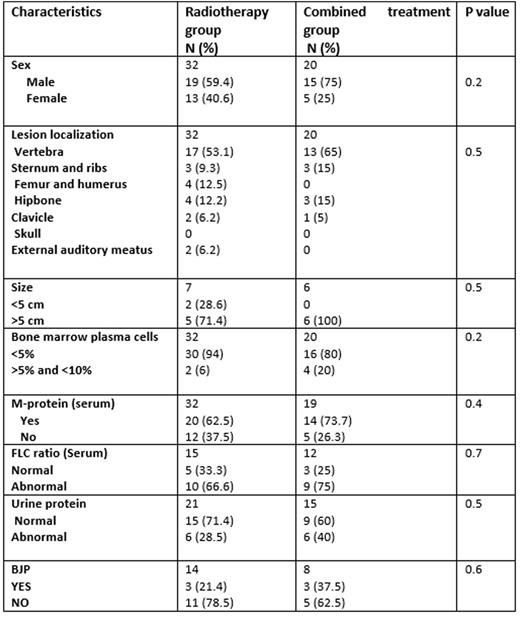
In this study, the diagnosis of SBP was based on criteria published in 2014 by the international myeloma working group (IMWG). All patients had a histological diagnosis based on biopsy-proven monoclonal plasma cell neoplasm. The solitary nature of the lesion was confirmed by MRI and/or TEP and/or CT scan. All patients had a normal marrow evaluation. We compared baseline characteristics and evolution between patients treated by radiotherapy alone and those treated with combined treatment (Table 1). Our primary study endpoint was progression-free survival (PFS) at 5 years.
Fifty-two patients with SBP, managed in 4 French centers between 1991 and 2015, were included. Twenty patients received combined treatment (adjuvant chemotherapy + radiotherapy) and 32 patients were treated by radiotherapy alone, according to each center policy. Chemotherapy regimen was heterogeneous between patients, and it was essentially based on Melphalan or Velcade or Thalidomide and corticosteroids. The median time of follow-up was 4.6 years (range 2.1-6.2 years). Relapse was recorded in 29 (56%) patients, and 25 (86.2%) patients developed MM. The mean time to develop MM was 3.3 +/- 4.9 years. The 5-year PFS rate in the radiotherapy group was 27.5% +/- 8.7%, while in the combined treatment group it was 53.6% +/- 16.2%, this difference was not significant (p=0.31). The 5-year overall survival (OS) rate in the radiotherapy group was 94% +/- 5.2 % versus 97 % +/- 4 % for the combined treatment group, (p =0.17).
Our results suggest that adjuvant chemotherapy has no impact on PFS and OS for the treatment of SBP and cannot prevent SBP progression to MM. Our results need to be confirmed in a larger cohort of patients. Moreover, prospective studies are mandatory to identify predictive factors to allow individualized therapy and to investigate new therapeutic strategies for SBP.
Moreau:Novartis: Honoraria; Amgen: Honoraria; Takeda: Honoraria; Bristol-Myers Squibb: Honoraria; Janssen: Honoraria, Speakers Bureau; Celgene: Honoraria. Decaux:The Binding Site: Other: supply of free light chain assays , Research Funding; SIEMENS: Honoraria, Other: supply of free light chain assays , Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal