Abstract
Background: Relapse of disease remains a frequent cause of mortality after allogeneic hematopoietic stem cell transplantation (HSCT). For patients with imminent relapse, therapeutic donor lymphocyte infusions (DLI) offer a treatment option for re-induction of complete remission of the underlying disease by employing the graft-versus-leukemia (GvL) effect. Moreover, DLI is used pre-emptively to prevent relapse in patients with high risk disease. DLI is given in increasing doses, adapted to clinical signs of graft-versus-host disease (GvHD) or molecular markers of disease, i.e. donor chimerism or minimal residual disease. There is a need for reliable monitoring of the immunologic response preceding the clinical outcome, enabling a precise administration of DLI dose escalation and thus possibly preventing overshooting immune reactions. With the advent of deep sequencing technology direct measure of high resolution T cell receptor (TCR) diversity can be used as a read-out of the immunologic response in the patient at the molecular level.
Aims: In this study we aim to elucidate whether TCR-diversity can serve as a biomarker for clinical outcome of DLI treatment.
Patients and Methods: We assessed 19 patients, who received DLI after HSCT. Therapeutic DLI was given in 14 patients and prophylactic DLI in 5 patients. The majority of patients (n=14) was treated for AML or MDS, 2 for ALL, and 3 for MPN. Peripheral blood samples for TCR-sequencing were obtained from the patient pre-DLI, at 2 and 4 weeks post DLI, and subsequently when available. Also, donor samples were collected. Donor's and patient's lymphocytes were FACS-sorted into CD8+ and CD4+ conventional (Tconv) and CD4+CD127-CD25+ regulatory T cells (Treg). Sorted subpopulations were subject to cDNA-based CDR3-region amplification by RACE-PCR allowing assessment of the entire TCR-β repertoire. Reliable generation of CDR3 amplicons was possible from as few as 4000 cells. These were then sequenced using the Illumina MiSeq platform. Reads were annotated by the IMGT.org database; further bioinformatics analyses included VDJtools and the tcR R-package.
Results: GvL response (remission or stable disease) could be achieved in 14/19 patients; 8 of these patients developed acute GvHD ≥III°. Flow cytometric analysis showed that the ratio between CD8+ and CD4+ Tconv in the DLI is predictive of response to DLI: DLIs containing a majority of CD4+ Tconv were associated with development of GvHD (n=8, CD8:CD4 = 35.3%:54.9%) , whereas patients responding to DLI with GvL only had received a higher proportion of CD8+ T cells (n=6, CD8:CD4 = 59.1%:29.5%); comparison of ratios met statistical significance (Mann Whitney test, p=0.0027).
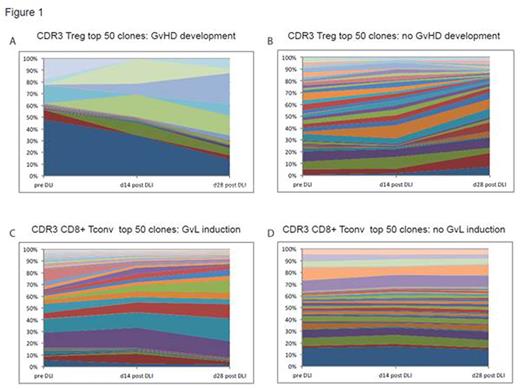
TCR sequencing revealed that the diversity of the TCR-repertoire seems to be predictive of the clinical course of the patient after DLI. GvHD development within 2 weeks of blood sampling time (n=4) was preceded by an increase of Treg repertoire diversity (assessed with inverse Simpson's diversity index, 1/Ds). Compared to the pre-DLI repertoire 1/Ds, mean increase was 143.67% vs. a 36.04% decrease in patients not developing GvHD (n=7; Mann Whitney test, p=0.02). Steroid treatment of GvHD (n=2) led to a mean decrease of 49.71% of Treg TCR 1/Ds compared to the previous time point. Analysis of GvL response revealed an association of remission induction with a trend towards decreased 1/Ds of the CD8+ TCR-repertoire (mean decrease of 27.66% at d28 after DLI compared to pre-DLI vs. 0.31% decrease in patients showing no GvL effect). Assessment of TCR CDR3 region clonotype expansion over time is shown for a patient responding with GvHD (Fig. 1A) and GvL (Fig. 1C) to DLI and a patient progressing without response to DLI (no GvHD Fig. 1B, no GvL Fig. 1D).
Conclusion: Our data indicate that TCR sequencing allows the assessment of TCR-diversity change as a surrogate parameter of DLI response. While an increase of Treg diversity seems to indicate the development of GvHD, repertoire compression of the CD8 compartment may be predictive of GvL response. Taken together, monitoring TCR repertoires may become a valuable predictive tool to improve DLI therapy and furthermore, analysis of expanding clonotypes after DLI enables identification of individual CDR3 sequences associated with DLI response.
Heuser:Pfizer: Research Funding; Novartis: Consultancy, Research Funding; BerGenBio: Research Funding; Karyopharm Therapeutics Inc: Research Funding; Tetralogic: Research Funding; Celgene: Honoraria; Bayer Pharma AG: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal