Abstract
Background: Sickle cell disease (SCD) is common throughout sub-Saharan Africa, where 75% of the 300,000 global births of affected children live. A significant proportion of these children die before adulthood, with preventable conditions such as pneumococcal infection. Introduction of neonatal screening in the developed world has cut down the mortality rate for SCD from 16% to >1% by allowing targeted interventions. A low-cost, rapid and point-of-care diagnostic test is needed to improve the feasibility of neonatal SCD screening in developing countries due to limited economic constraints. Lateral flow-immunoassay (LFIA) devices offer an attractive approach to develop such a test. LFIA tests are widely used for the diagnosis of infectious and non-infectious diseases in developing countries and offer advantages of stability, precise performance, interpretation by minimally trained users without requiring refrigeration during shipping and storage.
Methods: The conventional antibody (ab) based diagnostic approach to SCD is to utilize antibodies specific to the mutated hemoglobin (Hb), but such a strategy requires multiple antibodies, each specific to a given mutated form. To design a simple, cost-effective diagnostic, an alternative strategy of utilizing a single anti-human HBB ab that does not recognize any mutated form was adopted. In this device, a positive line would denote normal adult Hb and an absence of a positive line would denote a diagnosis of SCD. A number of monoclonal anti-human HBB abs were initially screened in direct enzyme-linked immunoassays (ELISA) using different purified hemoglobins (HbA0, HbS and HbA2) as antigens to identify candidates to develop the LFIA device. Based on the results from direct ELISAs, appropriate capture and detection ab pairs were generated to develop sandwich ELISA, which can be translated to a sandwich LFIA format. Results of ELISAs using pure Hbs were confirmed by using diluted peripheral blood samples from healthy and SCD patients. Strip-based LFIA was designed with 4 antibodies A) a device control using anti-mouse ab that confirms appropriate lateral flow B) a Hb control ab that recognizes all forms of HBB to confirm the presence of enough adult Hb in the sample, C) an HBB ab that only recognizes HbA0 but not HbS and HbA2 and D) a gold conjugated alpha chain specific ab as a labelled recognition element. The incorporation of gold nanoparticles as labels allows visual examination of colors at the test and control lines, resulting in qualitative or semi-quantitative analysis.
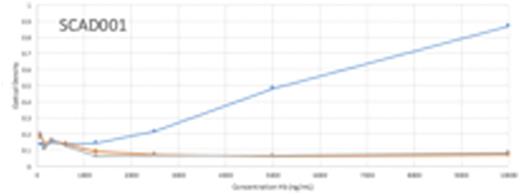
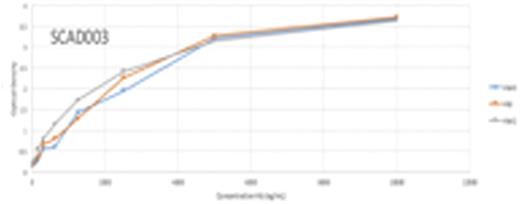
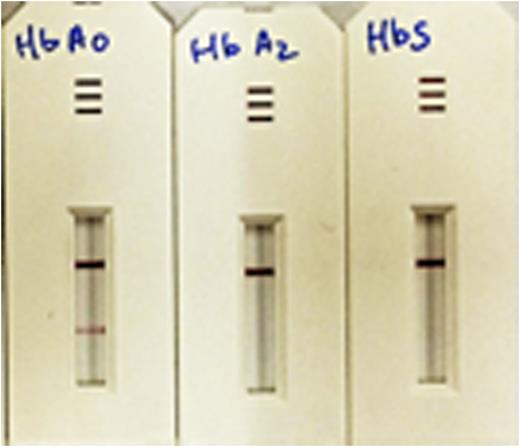
Results: The results from the direct ELISA screening identified two potential antibodies that can be used in the LFIA, SCAD001 and SCAD003. SCAD001 was the most differentiating with a 10-fold difference between HbA0 vs. HbA2 or HbS, which allows its use as a test line. The optical density (OD) reading for SCAD001 with HbA0 HbA2 and HbS were 0.867, 0.07 and 0.08, respectively (Figure 1a). SCAD003 was able to bind all three variants of Hb with high OD readings of 3.7, 3.6, and 3.7, respectively, suggesting its potential to be used as a gold conjugation ab. In the sandwich ELISA format, SCAD001 demonstrated excellent selectivity for HBA0 over other variants of Hb (1.4 vs .04 for HbA0 and HbS, respectively). A pilot LFIA test device was then designed using SCAD001. Representative results as shown in Figure 2 from an LFIA test using SCAD001 demonstrate that the positive line is evident with HbA0 but not with HbA2 and HbS. These devices are currently being scaled up to be evaluated in different test conditions. Results from the current experiments using peripheral blood samples from patients with different forms of SCD and a comparison of current standard of care diagnostic vs LFIA device will be included in the presentation.
Conclusion: These results demonstrate the feasibility of a novel approach of designing an LFIA device based on anti-HBB ab that selectively identifies normal Hb but not other variants. With its simple design and a single differentiating ab this device has the potential to be further developed as a neonatal screening test in low resource countries.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal