Natural killer (NK) cells are cytotoxic innate lymphoid cells, which play a major role in tumor surveillance. We have tested the safety and efficacy of allogeneic NK cell adoptive transfer from heathy haploidentical donors and demonstrate that in vivo expansion and persistence of the adoptively transferred NK cells at Day 14 after infusion correlates with 30-50% remission in patients with refractory AML. However, the factors that influence successful persistence of donor-derived NK cells are unclear. We hypothesized that recipient T cells play a role in the rejection of allogeneic NK cells and a correlation could exist between persistence of donor-derived NK cells and exhaustion in recipient T cells. T cell exhaustion, a well-established state of T-cell dysfunction occurring in response to chronic and continuous antigen stimulation, is well-documented in human cancer, and characterized by progressive and hierarchical loss of effector functions including sustained up-regulation and co-expression of multiple inhibitory receptors such as PD-1 and Tim-3 and altered expression of key transcription factors including the gain of Eomes and T-bet.
We used samples from a phase I/II trial of CD3/CD19 depleted, IL-15-activated, haploidentical donor NK cells delivered following conditioning with cyclophosphamide (50mg/kg) and fludarabine (35 mg/m2 x 3days) in adults with chemotherapy refractory AML. Patients received donor NK cells on Day 0 followed by 10 doses of recombinant human (rh) IL-15 (2 mcg/kg/day) manufactured by the NCI and delivered SQ on Days 1-5 and 8-12. A significant proportion of patients experienced donor NK cell expansion at Day 14 (expanders), but there were some that did not (non-expanders). Therapeutic benefit has only been noted among the expanders.
We examined samples from a total of 10 patients with refractory AML, 5 expanders and 5 non-expanders, along with their 10 respective donors. Cryopreserved patient PBMCs were thawed and rested overnight in RPMI-1640 with 2% FBS. The cells were stained for viability, for surface markers using antibodies against CD3, CD8, CD56, PD-1, and Tim-3, intracellularly stained for Eomes and T-bet.
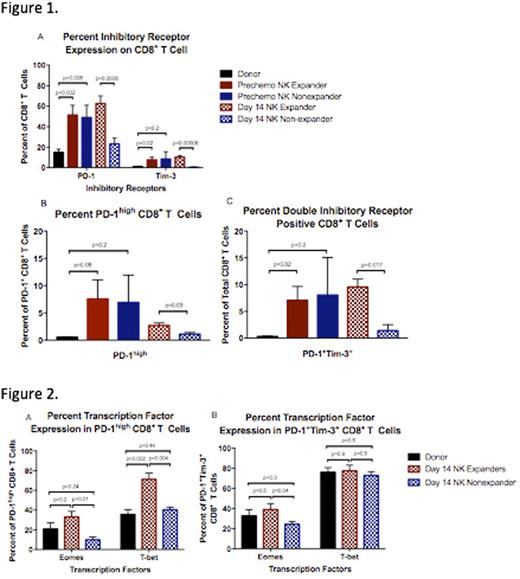
We evaluated CD8+ T cell expression of PD-1 and Tim-3, in expanders and non-expanders, prior to chemotherapy and at Day 14. Paired donor T cells from the non-mobilized apheresis products served as controls. Prior to chemotherapy, both patient groups had equivalently elevated expression of both PD1 and Tim-3 on CD8+ T cells. However, at Day 14, the expanders had persistence of PD-1 and Tim-3 while expression on non-expander CD8+ T cells fell to donor level (Figure 1A). Furthermore, expanders had a significantly higher proportion of CD8+ T cells that either co-expressed PD-1 and Tim-3 (p=0.017) or had a PD-1high phenotype (p=0.032) at Day 14, both of which are suggestive of an exhausted state, as opposed to an activated one (Figure 1B,C).
Next, we examined Eomes and T-bet expression in recipient T cells. While generally low among healthy T cell populations, as T cells become exhausted, they gain expression of these transcription factors. We looked specifically at the expression of these transcription factors in the recipient CD8+ T cell populations with the highest likelihood of being exhausted, i.e. those co-expressing PD-1 and Tim-3 or those with the PD-1high phenotype. Eomes expression in recipient PD-1high CD8+ T cells and in PD-1+Tim-3+ CD8+ T cells at Day 14 was significantly higher (p=0.01 and p=0.04, respectively) among expanders compared to non-expanders (Figure 2A,B). Likewise, T-bet expression was greater (p=0.004) among expanders in the PD-1high population (Figure 2A). There was no difference in the T-bet expression in PD-1+Tim-3+ CD8+ T cells between groups (Figure 2B).
While all patients with refractory AML receiving NK cell adoptive transfer had an elevated percentage of CD8+ T cells with an exhausted phenotype prior to therapy, only patients with donor-derived NK cell expansion had persistence of the exhausted T cell phenotype at Day 14. Thus, T cell mediated rejection is a major obstacle to overcome for successful adoptive NK cell transfer which could in part be aided by a link between recipient T cell exhaustion and expansion of NK cells. This might further suggest that IL-15 reverses T cell exhaustion among those who failed to achieve donor-derived NK cell expansion.
Miller:Fate Therapeutics: Consultancy, Research Funding; Oxis Biotech: Consultancy, Other: SAB.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal