Abstract
CONTEXT
Chronic Lymphocytic Leukemia (CLL) is a clonal proliferation of small mature B-cell lymphocytes diagnosed clinically when the peripheral blood clonal B lymphocyte count is persistently >5,000/mcL with distinctive immunological markers defined by co-expression of CD5 and CD23 with additional expression of CD19, CD20 (weak) with weak surface immunoglobulin expression, usually IgM heavy chain. Expression of CD79b and FMC7 is typically negative to weakly positive. With the recent advancement in cancer genetics and the continued understanding of the transforming events in CLL, the importance of the various somatic genomic aberrations has been well documented. Multiple studies have shown the clinical implications of these aberrations in terms of their prognostic and predictive relevance in clinical practice and these genomic aberrations are usually assessed clinically by cytogenetic analysis and FISH.
OBJECTIVE
We proposed this study to determine the incidence of the genomic aberrations in the CLL patients diagnosed at the Rose Cancer Treatment Center of the William Beaumont Hospital between 2010 and 2015 and to determine their impact on survival among the patients diagnosed during the study period. STUDY DESIGN
A retrospective review of all the patients diagnosed with CLL between 2010 and 2015 at the Rose Cancer Treatment Center was conducted with the assistance of the staff in the William Beaumont cancer registry office. We determined the demographic variables and analyzed the incidence of CLL among the subjects diagnosed within the study period. Data analysis was performed using SPSS 21 and Kaplan-Meier curves were plotted for survival analysis and log rank (Mantel-Cox) was used to compare these curves. 12-Month and 36-Month overall survival rates were analyzed by actuarial method. The distribution of the various genomic aberrations was determined using descriptive statistical analysis. RESULTS
A total of 151 patients were identified at the Rose Cancer Center of the William Beaumont Hospital during the study period. The median age at diagnosis was 74 years (range 38-101) of which 90 were male (59.6%) and 61 female (40.4%). One-hundred and twenty-four (82.1%) patients were white, six patients (4.0%) were African American, two patients (1.3%) were Asian and nineteen (12.6%) patients declined to identify their race.
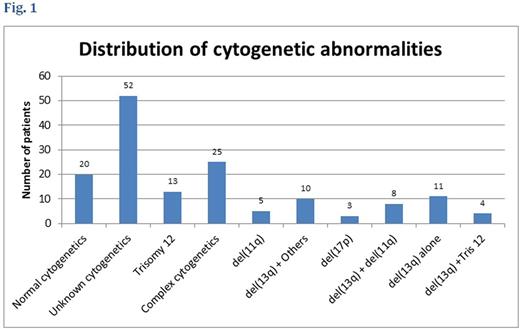
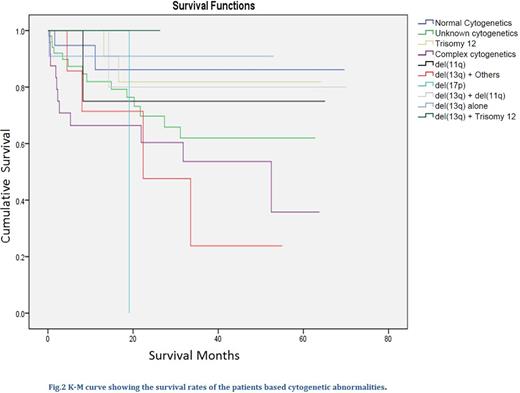
Analysis of cytogenetic distribution showed that, twenty patients (13.2%) had normal cytogenetic, eleven patients (7.3%) had del(13q) alone, eight (5.3%) had both del(13q) and del(11q), four patients (2.6%) had del(13q) plus trisomy 12 aberrations, ten (6.6%) patients had del(13q) and other karyotypes (TP53, RB1, trisomy 1q, del(6q23), unmutated IGHV). Thirteen patients (8.6%) had trisomy 12 abnormality, five patients (3.3%) had del(11q), three patients (2.0%) had del(17p), twenty-five patients (16.6%) had complex cytogenetic abnormalities and fifty-two (34.4%) patients cytogenetic were not checked (see Fig.1). The median follow-up duration for the cohort was 22.5 months (range 0 to 70 months). The survival rates at 12 months and 36 months for the cohorts based on cytogenetic are described in table 1 and fig.2.
CONCLUSION
Our study showed that majority of our patients (34.4%) did not have their cytogenetics checked at diagnosis, patients with del(13q) abnormality alone had the most favorable 36-Month overall survival rate and those with del(17p) fared worst with the most unfavorable outcome followed by patients with complex cytogenetic abnormalities. Presence of del(13q) with either del(11q) or trisomy 12 abnormalities appeared to ameliorate their poor and intermediate adverse prognostic effects, respectively.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal