Abstract
Background:
By combining the metabolic information of PET with the high soft tissue contrast of MRI, PET/MRI, most often performed with the glucose analogue [18F]fluorodeoxyglucose (FDG), has emerged as a powerful hybrid imaging modality for various oncologic conditions. Multiple myeloma (MM) is an attractive target for FDG-PET/MRI, as both MRI and FDG-PET/CT, when performed separately, have important limitations. For example, diffuse tracer uptake by normal marrow can obscure focal myeloma lesions on FDG-PET, especially in patients receiving marrow-stimulating agents (Hanrahan et al.,RadioGraphics, 2010), while the correlative CT images are less sensitive than MRI for subtle marrow infiltration (Baur-Melnyk et al., AJR, 2008). However, such abnormal marrow signal on MRI can persist despite clinical evidence of disease remission, while the resolution of FDG uptake by focal myeloma lesions closely correlates with clinical metrics of treatment response (Walker et al., Blood, 2004). Thus, FDG-PET/MRI, by interrogating both lesion metabolism and marrow replacement, has the potential to serve as a comprehensive imaging tool for MM and other plasma celldyscrasias (PCDs). In this study, we provide an account of our experience with FDG-PET/MRI for PCDs at a large academic center.
Methods:
Since 2012, 36 patients with clinical diagnoses of MM, smoldering MM (SMM), or monoclonalgammopathyof undetermined significance (MGUS) have undergone FDG-PET/MRI at our institution. Fourteen were imaged with both PET/CT and PET/MRI as part of a PET/MRI optimization research protocol, while 22 underwent PET/MRI as a standard-of-care clinical examination. An integrated SiemensBiographmMRwas used for all PET/MRI acquisitions, while PET/CT was performed on a SiemensBiograph40 ormCT. Our PET/MRI myeloma protocol, which extends from skull vertex to knees, generally included two-point Dixon images for attenuation correction,transaxialT2-weighted images (T2WIs), sagittal (spine only) andtransaxial(pelvis only) turbo spin echo (TSE) T1-weighted images (T1WIs), andtransaxialpost-contrast T1WIs.PET/MRI studies were interpreted jointly by a nuclear medicine physician and a radiologist specializing in MRI. Patient characteristics, clinical scenarios, and imaging findings were compiled and analyzed in aggregate.
Results:
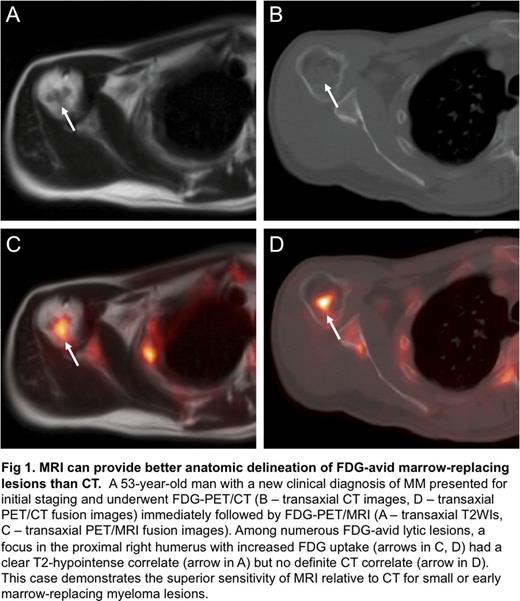
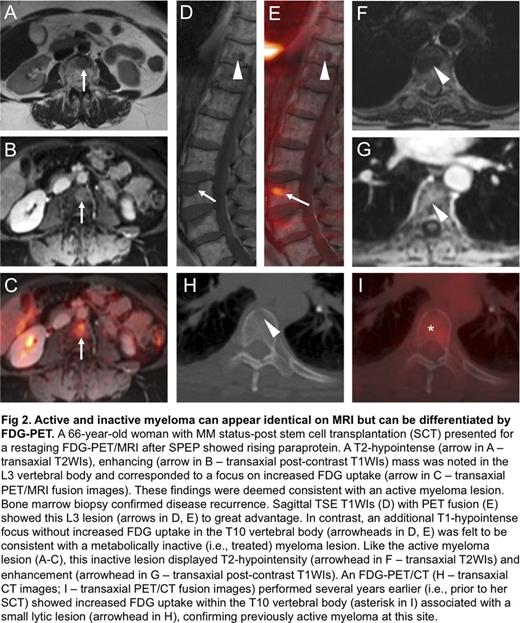
Among 15 patients with new clinical diagnoses of MM, 9 (60%) had FDG-avid bone lesions. In contrast, among the 10 patients with new clinical diagnoses of MGUS or SMM, none had FDG-avid bone lesions. Of the remaining 11 patients imaged for suspected recurrence or surveillance of previously treated MM, only 4 had FDG-avid bone lesions. Analysis of 20 FDG-avid bone lesions imaged on the same day with both PET/CT and PET/MRI revealed CT correlates for 15 lesions (75%) and MRI correlates for 14 lesions (70%). Notably, there were 5 spinal lesions in a single patient that had CT correlates without MRI correlates; however, no sagittal TSE T1WIs of the spine were acquired, reducing the sensitivity of MRI. Five of these 20 FDG-avid bone lesions were apparent on MRI but not CT (Fig 1). Using marrow biopsy and/or serum protein electrophoresis (SPEP) as a standard of reference, FDG-PET correctly differentiated metabolically active from inactive disease in 6 of 8 patients with previously treated MM. Active and inactive lesions were otherwise indistinguishable on MRI alone; both lesion types generally exhibited focal T2-hyperintensity and enhancement (Fig 2).
Conclusions:
FDG-PET/MRI is feasible for both the initial staging and subsequent treatment response assessment of PCDs. To maximize the clinical utility of PET/MRI relative to PET/CT for the anatomic depiction of marrow-replacing lesions, TSE T1WIs should be incorporated into standard protocols, especially for the spine and pelvis. The addition of FDG-PET to whole-body MRI is useful for differentiating metabolically active and inactive disease, which can look identical on MRI alone. PET/MRI also provides substantial radiation dose reductions relative to PET/CT, especially in patients with malignancies such as MM that require frequent follow-up imaging studies (Schafer et al., Radiology, 2014). Finally, new PET tracers under development, such as those targeting VLA-4, may further expand the role of PET/MRI in PCDs by improving sensitivity for early or limited disease (Soodguptaet al., JNM, 2016).
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal