Abstract
Introduction
While 50-60% of patients with diffuse large B-cell lymphoma (DLBCL) are cured with initial chemoimmunotherapy such as R-CHOP, many patients will relapse and require additional therapy. Historically, autologous stem cell transplant (ASCT) has been utilized in chemo-sensitive patients with relapsed DLBCL although the role of ASCT in patients who require > 1 salvage treatment to achieve remission is less defined due to concerns about the likelihood of long-term remission in that population. We evaluated the outcome of ASCT in patients who required >1 salvage therapy.
Methods
We included all patients undergoing ASCT for relapsed/refractory DLBCL at our site between 2005-2016 who received > 1 salvage treatment before transplant, with radiation therapy considered a salvage treatment if given after relapse but before ASCT. We collected demographic, clinical, laboratory and pathologic data on all patients. We defined progression-free survival (PFS) as time from ASCT to date of progression or death from any cause and overall survival (OS) as time from ASCT to date of death from any cause. Living patients were censored at the time of their last follow up. Univariate Cox proportional hazards models of PFS and OS were fit and Kaplan-Meier plots were developed to estimate the impact of variables of interest on survival.
Results
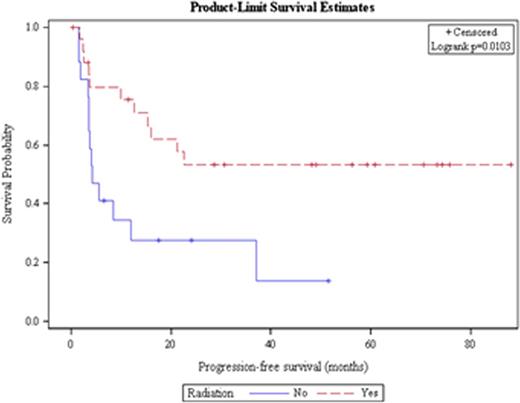
Out of 259 patients undergoing ASCT for DLBCL, 43 received > 1 salvage treatment, the median age was 51 years and 23 patients were male. Twenty patients had stage III/IV disease at diagnosis. The median time to relapse from the time of diagnosis was 9.3 months, and 42% of patients experienced a relapse > 12 months after diagnosis. Twenty-six patients (60%) received radiation as one of their salvage therapies, 25 patients (58%) received R-ICE as their first salvage therapy and 10 (23%) patients received R-ICE as their second salvage treatment. All patients received either 2 (n=39) or 3 (n=4) salvage therapies before ASCT, and the response to the initial salvage therapy received was CR in 4 patients, PR in 14 patients, SD in 1 patient, and PD in 14 patients, with initial response to salvage therapy missing in 10 patients. The median PFS for all patients was 15.9 months and the median OS was 57.2 months (Figure 1a). Receipt of radiation and having disease sensitive to treatment at the time of ASCT were the only factors associated with prolonged PFS and OS. Median PFS has not been achieved in patients who received radiation while patients who did not receive radiation had a median PFS of 4.2 months (HR = 0.36, p = 0.014; Figure 1b). Patients who had a chemo sensitive disease status at transplant had a median PFS of 22.6 months; however, patients with refractory disease at transplant only achieved a median PFS of 3.6 months (HR = 0.30, p=0.008). Remaining factors including conditioning regimen, time to relapse, and number of salvage therapies were not significantly associated with PFS or OS.
Conclusions
ASCT can result in prolonged PFS/OS in patients requiring > 1 salvage therapy especially in the case of sensitive disease. Radiation as an additional line of therapy is associated with improved PFS/OS, suggesting this can be included to induce remission in patients who fail to achieve CR with initial salvage treatment. While uncommon, patients with chemo-refractory disease can also have durable survival and should not be excluded from transplant.
Progression-free survival for all patients with DLBCL receiving > 1 salvage therapy.
Progression-free survival for all patients with DLBCL receiving > 1 salvage therapy.
Progression-free survival for all patients with DLBCL receiving > 1 salvage therapy based on receipt of radiation therapy.
Progression-free survival for all patients with DLBCL receiving > 1 salvage therapy based on receipt of radiation therapy.
Calzada:Seattle Genetics: Research Funding. Flowers:Spectrum, Janssen, Infinity, AbbVie, Acerta, Pharmacyclics, TG Therapeutics: Research Funding; Celgene Corporation: Consultancy, Honoraria; Optum Rx, Seattle Genetics, Genentech/Roche: Consultancy; Gilead: Consultancy, Research Funding; Millennium: Consultancy, Research Funding; Seattle Genetics: Research Funding. Cohen:Bristol-Myers Squibb: Research Funding; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Infinity: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pharmacyclics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Seattle Genetics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Millennium/Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal