Abstract
Introduction:
BGB-3111 is a potent, highly specific and irreversible Bruton tyrosine kinase (BTK) inhibitor, with greater selectivity than ibrutinib (IB) for BTK vs. other TEC- and HER-family kinases. We previously reported that BGB-3111 320mg daily (the RP2D, given as a single or split dose) achieved plasma concentrations 6- to10-fold higher than that of IB 560mg QD. Complete BTK occupancy in peripheral blood mononuclear cells (PBMCs) was observed in all pts treated in the dose-escalation (DEsc) component of the Phase 1 trial, and preliminary data suggested that >90% blockade was achievable in lymph nodes (LN). We now report the results of BTK occupancy analyses in LN specimens from a dedicated pharmacodynamics (PD) cohort and update safety and efficacy in patients with CLL/SLL.
Aims:
(1)To determine BTK occupancy in LN samples from pts receiving either a daily or twice-daily regimen; and (2) to define the safety profile and activity of BGB-3111 in pts with relapsed/refractory (R/R) or previously untreated CLL/SLL.
Methods:
This Phase 1 trial included a DEsc component in pts with R/R B-cell malignancies, and disease-specific expansion cohorts (ECs), including CLL/SLL, at the RP2D (320mg daily, given either QD or as a split BID dose). Additionally, in a PD cohort, pts with R/R B-cell malignancies were assigned to BGB-3111 160mg BID vs 320mg QD, with paired LN biopsies at baseline and at day 3 (pre-dose), in order to determine BTK occupancy in LN at the time of trough drug exposure. Adverse events (AEs) are reported per CTCAE v4.03, and response according to the 2012 clarification of the International Workshop on CLL criteria (for CLL pts) or the 2014 Lugano Classification (for SLL pts). BTK occupancy was determined by competitive fluorescent probe assay. The data cut-off for this report was 10 June 2016.
Results:
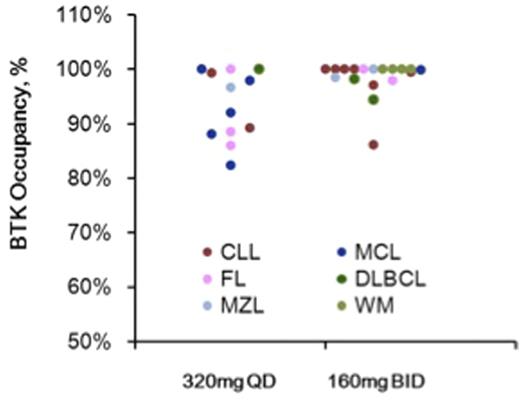
BTK Occupancy: 30 pts were evaluated for LN BTK occupancy (CLL, n=9; DLBCL, n=3; FL, n=5; MCL, n=6; MZL, n=3; WM, n=4), 23 pts were enrolled in the PD cohort; 7 patients in other ECs (160mg BID) consented to optional paired LN biopsies. BTK occupancy in LN by dose/schedule is shown in Figure 1. Median occupancy was 99.5% (160mg BID, n=18) vs 94.4% (320mg QD, n=12) (p=0.002, Wilcoxon). The proportion of pts with >90% occupancy was 94% (160mg BID) vs 58% (320mg QD) (p=0.027, Fisher exact). Occupancy did not appear to differ amongst histologic subtypes.
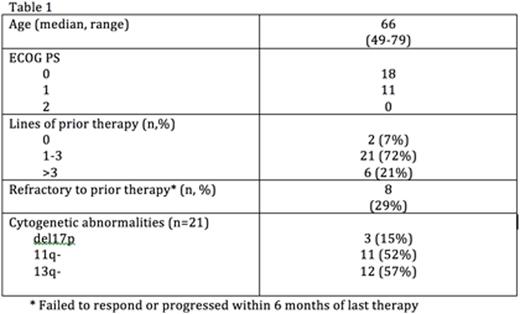
CLL/SLL Safety and Activity: As of 10 Jun 2016, 45 pts with CLL (n=42) or SLL (n=3) have been enrolled: 8 pts in DEsc (80mg QD [n=1], 160mg QD [n=2], 160mg BID [n=2], and 320mg QD [n=3]), and 37 in either the PD cohort or CLL/SLL EC (160mg BID, n=19; 320mg QD, n=18). 29 CLL/SLL pts are included in this analysis; 11 pts were excluded because of short (<12 weeks) follow-up, and 5 pts accrued at a single study site were excluded because of insufficient study documentation at baseline. Demographic and disease characteristics are shown in Table 1.
BGB-3111 was well tolerated with 69% subjects reporting no drug related AE >Gr 1 severity within the first 12 weeks of therapy. The most frequent AEs of any attribution were petechiae/ bruising (38%), upper respiratory tract infection (31%, all Gr 1/2), diarrhea (28%, all Gr 1/2), fatigue (24%, all Gr 1/2), and cough (21%, all Gr 1/2). Three SAEs were assessed as possibly related to BGB-3111 (Gr 2 cardiac failure, Gr 2 pleural effusion and Gr 3 purpura, all n=1). The case of Gr 3 purpura was the only major bleeding event reported. Atrial fibrillation (Gr 2) occurred in one pt. Three pts had temporary dose interruption for AE, and one pt discontinued BGB-3111 for AE.
After a median follow-up of 7.5 months (2.9-17.3 months), the response rate is 90% (26/29), with PR in 79% (23/29) and, PR-L in 10% (3/29), SD in 7% (2/29), and non-evaluable response in one pt who discontinued treatment prior to week 12. No instances of disease progression or Richter transformation have occurred.
Conclusions:
BGB-3111 is well-tolerated and highly active in R/R CLL/SLL. While trough BTK occupancy in lymph nodes was robust with either 320mg QD or 160mg BID dosing, complete and continuous occupancy (median 99.5%) was more frequently achieved with the 160mg PO BID regimen. Late stage clinical trials will determine if the optimized BTK occupancy with this regimen translates into improvements in disease control and reduced drug resistance.
Tam:janssen: Honoraria, Research Funding; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees; AbbVie: Honoraria, Membership on an entity's Board of Directors or advisory committees. Opat:Roche: Consultancy, Honoraria, Other: Provision of subsidised drugs, Research Funding. Gottlieb:Indee: Membership on an entity's Board of Directors or advisory committees; Celgene: Research Funding; Abbvie: Membership on an entity's Board of Directors or advisory committees. Simpson:Celgene, Roche, Janssen: Honoraria; Amgen Pharmaceuticals: Research Funding. Anderson:Walter and Eliza Hall Institute of Medical Research: Other: Walter and Eliza Hall Institute of Medical Research receives milestone payments for the development of venetoclax. Kirschbaum:Beigene: Employment. Wang:Beigene: Employment. Xue:Beigene: Employment. Yang:BeiGene: Employment. Hedrick:Beigene: Employment. Seymour:Gilead: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; AbbVie Inc.: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Genentech: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees. Roberts:Genentech: Patents & Royalties: Employee of Walter and Eliza Hall Institute of Medical Research which receives milestone payments related to venetoclax; Janssen: Research Funding; Genentech: Research Funding; AbbVie: Research Funding; Servier: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal