Abstract
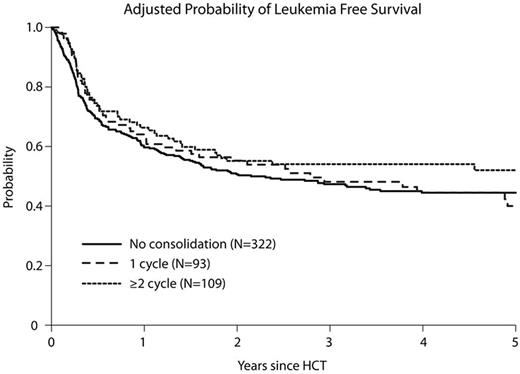
Allogeneic hematopoietic cell transplantation (alloHCT) is potentially curative for patients with ALL who achieve complete remission with upfront chemotherapy (CR1). However, the necessity of consolidation chemotherapy remains uncertain in patients with an available donor undergoing alloHCT, even if the goal is to reduce the detectable disease burden prior to alloHCT. We therefore compared clinical outcomes of 524 adult patients with ALL in CR1 who received ≥2 cycles (n=109) vs. 1 cycle (n=93) vs. no (n=322) consolidation chemotherapy prior to myeloabalative alloHCT from 2008-2012. The median follow up of survivors was 59 (6-78) months. All three consolidation groups had similar patient, disease and transplant characteristics, but no consolidation patients were older, took longer to achieve CR1 and less frequently received CNS prophylaxis or maintenance chemotherapy prior to alloHCT. In contrast, fewer with 1 cycle consolidation had prior comorbidities. Time to alloHCT was longer with increasing cycles of consolidation. Only a minority had either cytogenetic or molecular detectable disease in their pre-HCT CR1 assessment; and it was similar in each consolidation cohort. For ≥2 cycles, 1-cycle, no consolidation groups, the adjusted 3-year cumulative incidence of relapse was 20%, 27% and 22% and 1-year transplant-related mortality (TRM) was 16%, 18% and 23%, respectively (all p-values > 0.4). Similarly, adjusted 3-year leukemia-free survival (LFS) was 54%, 48% and 47% (Figure), while overall survival (OS) was 63%, 59% and 54%, respectively (all p-values > 0.3). Consolidation did not influence acute or chronic GVHD risks. Multivariable regression analysis adjusted for recipient age, Karnofsky performance status, time to CR1, graft source, donor type and recipient CMV serostatus, confirmed that consolidation chemotherapy was not prognostic of LFS (RR=1.20, 95% CI 0.86-1.67; p=0.28 for no consolidation; RR=1.18, 95% CI 0.79-1.76; p=0.41 for 1 cycle; ≥2 cycles=reference). Similarly, consolidation was not associated with OS, relapse, TRM or GVHD. We conclude that consolidation chemotherapy does not benefit adult ALL patients with readily available donor who undergo myeloablative alloHCT in CR1.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal